Deep brain stimulation and mental health: Novel experimental therapy gives new hope to patients with treatment-resistant depression

Shannon Paige McGann felt immediate relief after her deep brain stimulation procedure. (Photo credit: Maricruz Kwon/UTHealth)
An experimental treatment option now available for the first time in North America for people struggling with treatment-resistant depression (TRD) has researchers optimistic.
The Center of Excellence on Mood Disorders at The University of Texas Health Science Center at Houston (UTHealth) is conducting a clinical trial pilot study for a procedure called deep brain stimulation (DBS) for patients with chronic TRD by targeting an area called the medial forebrain bundle — an area of the brain thought to be important for reward processing. The trial is approved by the U.S. Food and Drug Administration.
DBS has actually been used for decades to help Parkinson’s patients whose tremors did not improve with medication, says Jair C. Soares, MD, PhD, director of the Center of Excellence on Mood Disorders and one of the researchers conducting the trial. DBS targeting the medial forebrain bundle has also been successfully used, on an experimental basis, to treat TRD in Germany.
Shannon Paige McGann, a patient in her late 40s enrolled in the pilot study, describes the treatment as “a miracle.” “I felt like someone turned on a light bulb in my brain. Everything is brighter. It changed my life,” she says.
Since she was a child, McGann has suffered from depression off and on. She was diagnosed with depression as a college freshman and was stabilized with medications. She went on to become a teacher for seven years, but after getting a new teaching position, her depression returned severely.

Shannon Paige McGann has experienced depression on and off for most of her life. (Photo provided by Shannon Paige McGann)
After that, McGann switched careers and excelled in pharmaceutical sales. Then, in 2007, her mother died and her boyfriend broke up with her — and she hit a new low. “It was a chore to get out of bed; I would lay in bed for days,” she recalls.
Thinking another change might be beneficial, she moved from Virginia to Houston in September 2016 to take a new job. However, she wasn’t able to handle it. At that point, no medications were working for her nor did electroconvulsive therapy.
Since these treatments weren’t effective, McGann says one of her doctors in Virginia told her about UTHealth performing the DBS trial and recommended it to her. The small trial, which is enrolling 10 patients, is studying whether DBS of the medial forebrain bundle is safe and effective.
What deep brain stimulation entails
DBS involves having a neurosurgeon permanently implant two electrodes that each have four small contacts, which deliver electrical stimulation to the target area, in this case, the medial forebrain bundle.
McGann says she wasn’t scared to undergo the treatment. “I had exhausted all other options,” she says.
Candidates for the treatment undergo a screening evaluation to determine if they meet the criteria for the study. This includes a thorough examination of their medical history, neuropsychological testing, bloodwork, drug screening and a physical. Patients in the trial undergo the procedure at Memorial Hermann-Texas Medical Center.
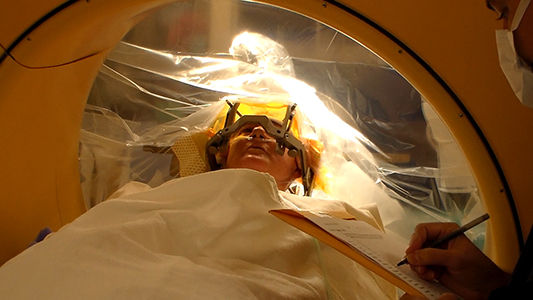
In this photo of Shannon Paige McGann’s deep brain stimulation procedure, Dr. Jair Soares asks her questions to assess her mood. (Photo provided by Department of Psychiatry and Behavioral Sciences at McGovern Medical School at UTHealth)
The procedure involves having the surgeon make an incision across the front of the head and drilling two holes down to the skull. Then, the surgeon places the electrodes and turns them on. The patient is kept awake during this time, but they don’t experience any pain because local anesthetic is used, and there are no pain receptors in the brain, says Albert Fenoy, M.D., associate professor at the Vivian L. Smith Department of Neurosurgery at McGovern Medical School at UTHealth and a member of the Mischer Neuroscience Institute at Memorial Hermann-Texas Medical Center.
“Patients need to be awake because we ask them questions to assess their mood and feelings while we test the electrodes at different spots in order to find the one which improves mood to the highest extent,” Fenoy says. “We will note changes in the patient’s energy or motivation, such as speaking more freely, smiling or laughing. That indicates that we are in the right spot. It is amazing to see, because it is provoked by the stimulation.”
Next, the patient is put to sleep and a wire that connects the electrodes and battery pack (called the neurostimulator) is implanted underneath the skin. The neurostimulator — the system’s power source — is placed inside a metal case below the clavicle. Similar to a small pacemaker, models range from two to three inches in diameter and are about 1/2 inch thick. The battery produces the electrical impulses needed to stimulate the electrodes.
The battery will need to be replaced approximately every three years, Fenoy says, which requires a minor surgical procedure.
After the surgery
McGann says she felt immediate relief when the surgery was over. She stayed in the hospital one night and went home the next day. “I remember wanting to do things, like going to the pool at my apartment complex, which I had no desire to do before,” she says.
Follow-up care includes having the patient meet with a neurosurgeon regarding their surgery and seeing a psychiatrist who monitors improvement in depressive symptoms every week by having them rate their level of depression. Patients also have a neuropsychological evaluation once a year to ensure that DBS is not interfering with brain functions, such as memory. The study follows patients for at least two years after surgery.
Patients may not experience immediate symptom suppression from DBS. Frequent, non-invasive adjustment to the stimulation parameters may be required. “The procedure is very experimental, so there’s no guarantee that someone will get better,” says Soares, who serves as professor and Pat R. Rutherford, Jr. Chair in Psychiatry at McGovern Medical School at UTHealth.
“But the goal is to see patients get better,” Soares adds. “Because depression is a chronic condition, patients may have still bumps along the road. But hopefully their lows won’t be as low, and they won’t last as long. We hope to see an uptrend in the patient’s mood, and that they have normal energy and enthusiasm for life.”
A look at past research
Soares notes that in past decades DBS treatment was used to treat patients with depression who didn’t respond to commonly available treatments — but different areas of the brain were targeted and it didn’t prove to be effective.
Then, during a preliminary study published in 2013 and conducted in Germany at University Hospital Bonn, researchers targeted a new area of the brain — the medial forebrain bundle — with success. Six of the seven patients in the study experienced a positive response for at least 24 months after their surgery.
Inspired by the German study, Soares paired up with the Vivian L. Smith Department of Neurosurgery at McGovern Medical School to design their own trial. To date, UTHealth has performed nine surgeries, with promising results. “We are very impressed with the results so far,” he says. “Patients are functioning better. Some are now working full-time and are enjoying life with their families.”
Looking ahead
If the trial continues to be successful, UTHealth researchers plan to ask the U.S. Food and Drug Administration to approve a study with a larger group of patients over a longer period of time. “For now, we are just trying to replicate and expand what our German colleagues reported,” Soares says.
“To find something that would help people who have run out of hope would be a major treatment advancement for TRD,” Soares adds.
McGann says she is so grateful for the opportunity to have had this surgery and for the great team of doctors. “The experience has been wonderful,” she says. “I highly recommend it.”
The UTHealth study has been conducted with funding from the Dunn Foundation of Houston.
The UTHealth Center of Excellence on Mood Disorders is currently enrolling patients for a clinical trial studying the safety and efficacy of deep brain stimulation (DBS) on treatment-resistant depression (TRD). Patients must be between the ages of 22 and 65 and diagnosed with TRD. DBS consists of a neurosurgeon implanting a small electrode that delivers electrical stimulation to an area of the brain that is responsible for symptoms such as feelings of sadness or guilt, fatigue, loss of interest in favorite activities, issues with sleeping and/or suicidal thoughts. For more information about the clinical trial and eligibility requirements, call 713-486-2627 or email utmooddisorders@uth.tmc.edu.
Written by: Karen Appold