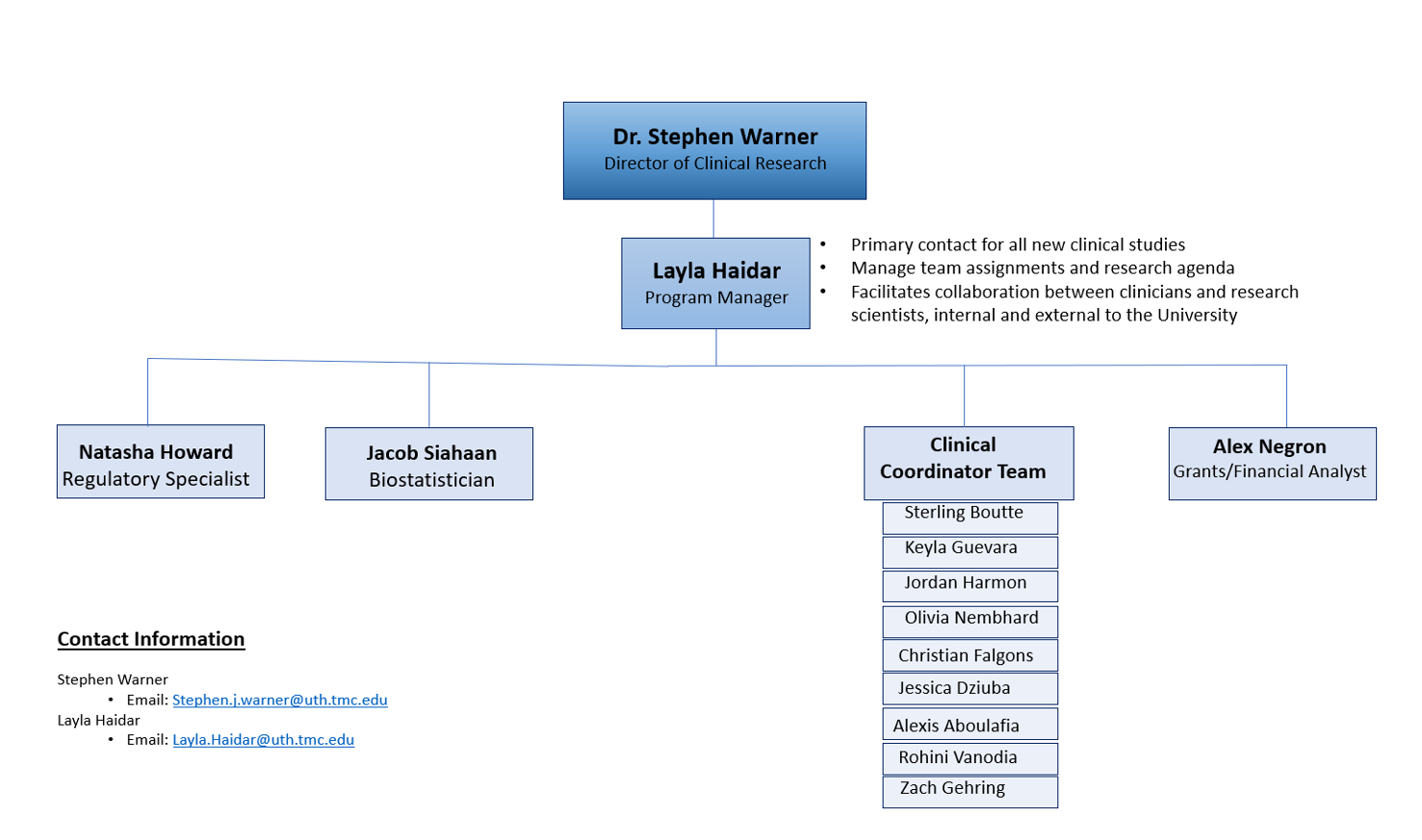
Clinical Research Section
Clinical Research Team

Stephen J. Warner, M.D., Ph.D.
Director of Clinical Research
Layla Haidar, MPH
Program Manager
Natasha Howard, BS
Regulatory Specialist
Jacob Siahaan, MS
Statistician
Alex Negron
Financial Analyst
Sterling Boutte, BS
Clinical Coordinator
Keyla Guevara
Clinical Coordinator
Jordan Harmon, BS
Clinical Coordinator
Past grant support:
- “A Pragmatic Randomized Trial Evaluating Pre-Operative Antiseptic Skin Solutions in Fractured Extremities”
PI: Warner, Stephen
Federal Support, METRC / DoD / University of Maryland / JHU
$151,999 2017 – 2022
- “REPAIR: Rehabilitation Enhanced by Partial Arterial Inflow Restriction | patient care (fixed fee)”
PI: Warner, Stephen
Federal Support, METRC / DoD / JHU
$160,494 2018 – 2023
- “Effect of Early Weight Bearing on Rehabilitation”
PI: Gary, Joshua
Federal Support, METRC / DoD / JHU
$43,306 2018 – 2020
- “A Safety and Technical Feasibility Evaluation of the Curvafix Intramedullary RodscrEw System for FixaTion Of Pelvic and AcetabulaR FracturEs – RESTORE Study”
PI: Warner, Stephen
Industry Support, Curvafix Inc
$53,901 2019 – 2011
- “Complications and Safety of Blood Clot Prevention Medicines Used in Orthopedic Trauma Patients”
PI: Warner, Stephen
Federal Support, METRC / JHU
$522,324 2017 – 2022
- “A Multi Center Prospective Observational Study of Nerve Repair and Reconstruction Associated with Major Extremity Trauma”
PI: Gary, Joshua
Federal Support, METRC / DoD / JHU
$44,800 2016 – 2021
- “An ACDF Multi-center Study Using ViviGen Cellular Bone Matrix”
PI: Prasarn, Mark
Industry Support, Johnson & Johnson Services Inc.
$23,900 2016 – 2021
- “A Phase 2, Randomized, Double-Blind, Placebo-Controlled Efficacy and Safety Study of Palovarotene in Subjects with Multiple Osteochondromas”
PI: Conrad, Ernest
Industry Support, PPD Investigator Services LLC
$305,762 2018 – 2022
- “Continuation of the Transtibial Amputation Outcomes Study (TAOS)”
PI: Gary, Joshua
Federal Support, METRC / DoD / JHU
$24,225 2017 – 2021
Current grant support:
- “The Major Extremity Trauma and Rehabilitation Research Consortium | PRECISE, EMSBIND, CBPT, AlterG”
$726,104 2017 – Current
Federal Support, METRC
PI: Warner, Stephen
- “The Mobility Toolkit: Electronically Augmented Assessment of Functional Recovery Following Lower-Extremity Trauma”
PI: Warner, Stephen
Federal Support, METRC
$72,125 2018 – Current
- “TOBRA – Novel Topical Antibiotic Therapy to Reduce Infection after Operative Treatment of Fractures at High Risk of Infection: A Multicenter Randomized, Controlled Trial”
PI: Warner, Stephen
Federal Support, METRC
$14,621 2020 – Current
- “SEXTANT: Title Evaluation of a New Strategy for Protocolized Antibiotic Care for Severe Open Fractures”
PI: Warner, Stephen
Federal Support, METRC
$7,076 2021 – Current
- “Effect of NSAIDs on Union, Opioid Utilization and Pain Management for Tibia Fractures: A Pragmatic, Randomized Controlled Trial”
PI: Warner, Stephen
Federal Support, METRC
$80,217 2020 – Current
- “Subacromial Bursa Implantation After Rotator Cuff Repair – A Randomized Controlled Trial”
PI: Gregory, James
Industry Support, Arthrex Inc.
$69,420 2021 – Current
- “Proximal Humerus Fixation with the Conventus CAGETM PH Device: A Post-Approval Observational Data Collection Study, A Single Center Study, Memorial Hermann”
PI: Gregory, James
Industry Support, Conventus Orthopaedics, Inc.
$96,335 2017 – Current
- “OXINIUM DH Total Hip Replacement System in Subjects with Non-Inflammatory Arthritis”
PI: Mathis, Kenneth
Industry Support, Smith & Nephew Inc.
$45,013 2019 – Current
- “IlluminOss(R) Device Global Registry – A Prospective, Post-Market, Multi-Center Evaluation of the Clinical Outcomes of the IlluminOss Device”
PI: Harvin, William
Industry Support, IlluminOss Medical Inc.
$27,953 2022 – Current
- “CIVO(R) Microdose Injections of Anti-Cancer Therapies”
PI: Conrad, Ernest
Industry Support, Presage Biosciences, Inc.
$48,815 2021 – Current
- “Evaluation of a Steady State Visual Evoked Potentials (SSVEP) in mild Traumatic Brain Injury (mTBI)”
PI: Ott, Summer
Industry Support, HeadsafeIP Pty Ltd
$37,635 2021 – Current
- “Tornier PERFORM Stemless Reverse IDE Study”
PI: Gregory, James
Industry Support, Stryker Orthopaedics
$17,557 2022 – Current
- “A Phase 1b Open-Label, Dose-Escalating Study to Evaluate the Safety and Tolerability of PLG0206 in Patients Undergoing Debridement, Antibiotics, and Implant Retention (DAIR) for Treatment of a Periprosthetic Joint Infection (PJI) Occurring After Total Knee Arthroplasty (TKA)”
PI: Rodriguez-Quintana, David
Industry Support, Peptilogics Inc.
$28,921 2022 – Current
- “Double Blinded Prospective Pilot Study to Determine the Safety and Effectiveness of a Connective Tissue Allograft (Active Matrix¿) vs. Standard of Care in Adhesive Capsulitis of the Shoulder”
PI: Berkman, Eric
Industry Support, Skye Biologics Holdings, LLC
$39,534 2022 – Current
- “Multicenter, Prospective Post-Market Clinical Follow-Up Study of the G7(R) Freedom Constrained Vivacit-E(R) Liner”
PI: Rodriguez-Quintana, David
Industry Support, Zimmer Biomet Inc.
$11,011 2023 – Current
- “Sacral Fracture Fusion/Fixation for Rapid Rehabilitation”
PI: Prasarn, Mark
Industry Support, SI-BONE, Inc.
$9,711 2023 – Closing Early
- “A Multi-Centre Study in Patients Undergoing Total Knee Replacement with Smith+Nephew Porous Total Knee Arthroplasty (TKA) System”
PI: Mathis, Kenneth
Industry Support, Smith & Nephew Inc.
10,070 2023 – Current
Recent publications
- Worsham, J., Lowe, W. R., Copa, D., Williams, S., Kleihege, J., Lauck, K., … & Bailey, L. (2019). Subsequent surgery for loss of motion after anterior cruciate ligament reconstruction does not influence function at 2 years: a matched case-control analysis. The American Journal of Sports Medicine, 47(11), 2550-2556.
- Castillo, R. C., Huang, Y., Scharfstein, D., Frey, K., Bosse, M. J., Pollak, A. N., … & Major Extremity Trauma Research Consortium. (2019). Association between 6-week postdischarge risk classification and 12-month outcomes after orthopedic trauma. JAMA surgery, 154(2), e184824-e184824.
- Rogers, N. B., Hartline, B. E., Achor, T. S., Kumaravel, M., Gary, J. L., Choo, A. M., … & Warner, S. J. (2020). Improving the diagnosis of ipsilateral femoral neck and shaft fractures: a new imaging protocol. JBJS, 102(4), 309-314.
- Campbell, S. T., Lim, P. K., Kantor, A. H., Gausden, E. B., Goodnough, L. H., Park, A. Y., … & Gardner, M. J. (2020). Complication rates after lateral plate fixation of periprosthetic distal femur fractures: a multicenter study. Injury, 51(8), 1858-1862.
- Doerre, T., Hallwachs, A. J., Fullick, R., & Lowe, W. R. (2020). Repair of an acute latissimus dorsi tendon rupture using bicortical button fixation. Techniques in Shoulder & Elbow Surgery, 21(3), 70-75.
- Garner, M. R., Warner, S. J., Heiner, J. A., Kim, Y. T., & Agel, J. (2020). Soft tissue management in open tibial shaft fractures: a comparison of institutional preferences and resultant early clinical outcomes. Bone & Joint Open, 1(8), 481-487.
- Warth, R. J., Zandiyeh, P., Rao, M., Gabr, R. E., Tashman, S., Kumaravel, M., … & Harner, C. D. (2020). Quantitative assessment of in vivo human anterior cruciate ligament autograft remodeling: a 3-dimensional UTE-T2* imaging study. The American Journal of Sports Medicine, 48(12), 2939-2947.
- Warth, R. J., Shupe, P. G., Gao, X., Syed, M., Lowe, W. R., Huard, J., & Harner, C. D. (2020). Fibrin clots maintain the viability and proliferative capacity of human mesenchymal stem cells: An in vitro study. Clinical Orthopaedics and Related Research, 478(3), 653.
- Suchting, R., Kapoor, S., Mathis, K. B., & Ahn, H. (2020). Changes in experimental pain sensitivity from using home-based remotely supervised transcranial direct current stimulation in older adults with knee osteoarthritis. Pain Medicine, 21(11), 2676-2683.
- Ahn, H., Galle, K., Mathis, K. B., Miao, H., Montero-Hernandez, S., Jackson, N., … & Pollonini, L. (2020). Feasibility and efficacy of remotely supervised cranial electrical stimulation for pain in older adults with knee osteoarthritis: A randomized controlled pilot study. Journal of Clinical Neuroscience, 77, 128-133.
- Bailey, L., Weldon, M., Kleihege, J., Lauck, K., Syed, M., Mascarenhas, R., & Lowe, W. R. (2021). Platelet-Rich Plasma Augmentation of Meniscal Repair in the Setting of Anterior Cruciate Ligament Reconstruction. The American Journal of Sports Medicine, 49(12), 3287-3292.
- Presley, J., Bailey, L., Maloney, K., Duncan, B., Reid, M., Juneau, C., & Lowe, W. R. (2021). The influence of mode-of-injury on psychological readiness for return-to-sport following anterior cruciate ligament reconstruction: a matched-controlled study. International journal of sports physical therapy, 16(1), 177.
- O’Toole, R. V., Stein, D. M., Frey, K. P., O’Hara, N. N., Scharfstein, D. O., Slobogean, G. P., … & Castillo, R. C. (2021). Protocol: PREVENTion of CLots in Orthopaedic Trauma (PREVENT CLOT): a randomised pragmatic trial protocol comparing aspirin versus low-molecular-weight heparin for blood clot prevention in orthopaedic trauma patients. BMJ Open, 11(3).
- Park, A. Y., & Smith, D. W. (2021). Volar Approach to the First Extensor Compartment for Surgical Treatment of DeQuervain Tenosynovitis. Techniques in Hand & Upper Extremity Surgery, 25(2), 108-110.
- Maxwell, G. T., Warth, R. J., Amin, A., Darlow, M. A., Bailey, L., Lowe, W. R., & Harner, C. D. (2021). Multiple ligament knee injuries: Does the knee dislocation classification predict the type of surgical management?. The Journal of Knee Surgery, 34(03), 273-279.
- Lin, C. A., O’Hara, N. N., Sprague, S., O’Toole, R. V., Joshi, M., Harris, A. D., … & PREP-IT Investigators. (2021). Low Adherence to Recommended Guidelines for Open Fracture Antibiotic Prophylaxis. JBJS, 103(7), 609-617.
- Rogers, N. B., Karam, W. N., Kumaravel, M., Warner, S. J., & Gary, J. L. (2021). Dual-Energy CT to Diagnose Occult Femoral Neck Fracture in MRI-Contraindicated Patient: A Case Report. JBJS Case Connector, 11(4), e21.
- Garner, M. R., Warner, S. J., Heiner, J. A., Kim, Y. T., & Agel, J. (2021). Evaluation of the orthopaedic trauma association open fracture classification (OTA-OFC) as an outcome prediction tool in open tibial shaft fractures. Archives of Orthopaedic and Trauma Surgery, 1-5.
- Han, S., Rodriguez-Quintana, D., Freedhand, A. M., Mathis, K. B., Boiwka, A. V., & Noble, P. C. (2021). Contemporary robotic systems in total knee arthroplasty: A review of accuracy and outcomes. Orthopedic Clinics, 52(2), 83-92.
- O’Toole, R. V., Joshi, M., Carlini, A. R., Murray, C. K., Allen, L. E., Huang, Y., … & Major Extremity Trauma Research Consortium. (2021). Effect of intrawound vancomycin powder in operatively treated high-risk tibia fractures: a randomized clinical trial. JAMA surgery, 156(5), e207259-e207259.
- O’Toole, R. V., Joshi, M., Carlini, A. R., Murray, C. K., Allen, L. E., Huang, Y., … & Major Extremity Trauma Research Consortium. (2021). Effect of intrawound vancomycin powder in operatively treated high-risk tibia fractures: a randomized clinical trial. JAMA surgery, 156(5), e207259-e207259.
- Ebaugh, M. P., McGarvey, W. C., Penner, M. J., & Berlet, G. C. (2021). Revision Total Ankle Arthroplasty. In Primary and Revision Total Ankle Replacement(pp. 421-446). Springer, Cham.
- Rennard, J., Rogers, N., Achor, T., Kumaravel, M., Gary, J., Choo, A., … & Warner, S. J. (2022). The Impact of Magnetic Resonance Imaging on the Diagnosis of High-Energy Ipsilateral Femoral Neck and Shaft Fractures. Journal of Orthopaedic Trauma, 36(2), 93-97.
- Rigsby, V. P., Shaw, J. C., Stankaitis, C. N., Kleihege, J. L., Lowe, W. R., Bailey, L., & Higbie, S. R. (2022, February). Return-to-Sport Outcomes Following ACL Reconstruction with Concomitant Meniscus Allograft Transplantation Versus Isolated ACL Reconstruction. In 2022 Combined Sections Meeting (CSM). APTA.
- McKinley, T. O., Gaski, G. E., Billiar, T. R., Vodovotz, Y., Brown, K. M., Elster, E. A., … & Castillo, R. C. (2022). Patient-Specific Precision Injury Signatures to Optimize Orthopaedic Interventions in Multiply Injured Patients (PRECISE STUDY). Journal of Orthopaedic Trauma, 36, S14-S20.
- Heiner, J. A., Banner, K. A., Wu, V. J., Achor, T. S., Gary, J. L., Munz, J. W., … & Warner, S. J. (2022). The Injury Characteristics of Open Pilon Fractures Predictive of Complications. Injury.
- Ebaugh, P., Alford, T. H., Davis, E., Kutzarov, K. J., Greaser, M. C., & McGarvey, W. C. (2022). Patient Reported Outcome Measures (PROMIS) of Primary Total Ankle Arthroplasty in Patients Under 50 Years of Age. Foot & ankle orthopaedics, 7(1), 2473011421S00184.
- Kellam, J. F. (2022). Are Bone Metabolism and Bone Material Strength Evaluations Worthwhile?: Commentary on an article by Tamara D. Rozental, MD, et al.:“Longitudinal Changes in Serum Markers of Bone Metabolism and Bone Material Strength in Premenopausal Women with Distal Radial Fracture”. JBJS, 104(1), e3.
- Vemulapalli, K. C., Pechero, G. R., Warner, S. J., Achor, T. S., Gary, J. L., Munz, J. W., … & Routt Jr, M. L. C. (2022). Is retrograde nailing superior to lateral locked plating for complete articular distal femur fractures?. Injury, 53(2), 640-644.