SEGA and EFIC
Whenever patients present to the Emergency Department with a large vessel occlusion, they are often unable to provide consent and are thereby typically excluded from emergency research participation – breaching the ethical principle of justice. With the design of the SEGA Trial, we came to the decision that we needed to implement an EFIC strategy due to the immediate action required in patient intervention.
The Food and Drug Administration has issued regulations for Exception from Informed Consent (EFIC) to facilitate research on potentially life-saving interventions in emergencies. Knowing we would need to implement this regulation to ensure efficient patient enrollment, we came up with a novel approach to gain EFIC approval with our Institutional Review Board (IRB).
During the recruitment of various centers, we discovered many obstacles in gaining EFIC approval. Due to the emergent nature of EVT, there is sometimes no LAR present to enable consent. With no EFIC protocol present, the concern is that centers will not be able to enroll patients, or have an extremely minimal amount of enrollment due to a lack of consent. However, based on the enrollments at sites that have an EFIC protocol, 38.4% of subjects were enrolled with patient or LAR consent. Meaning, that even if participating centers are unable to obtain an approvable EFIC protocol, they are still be able to enroll a significant amount of cases.
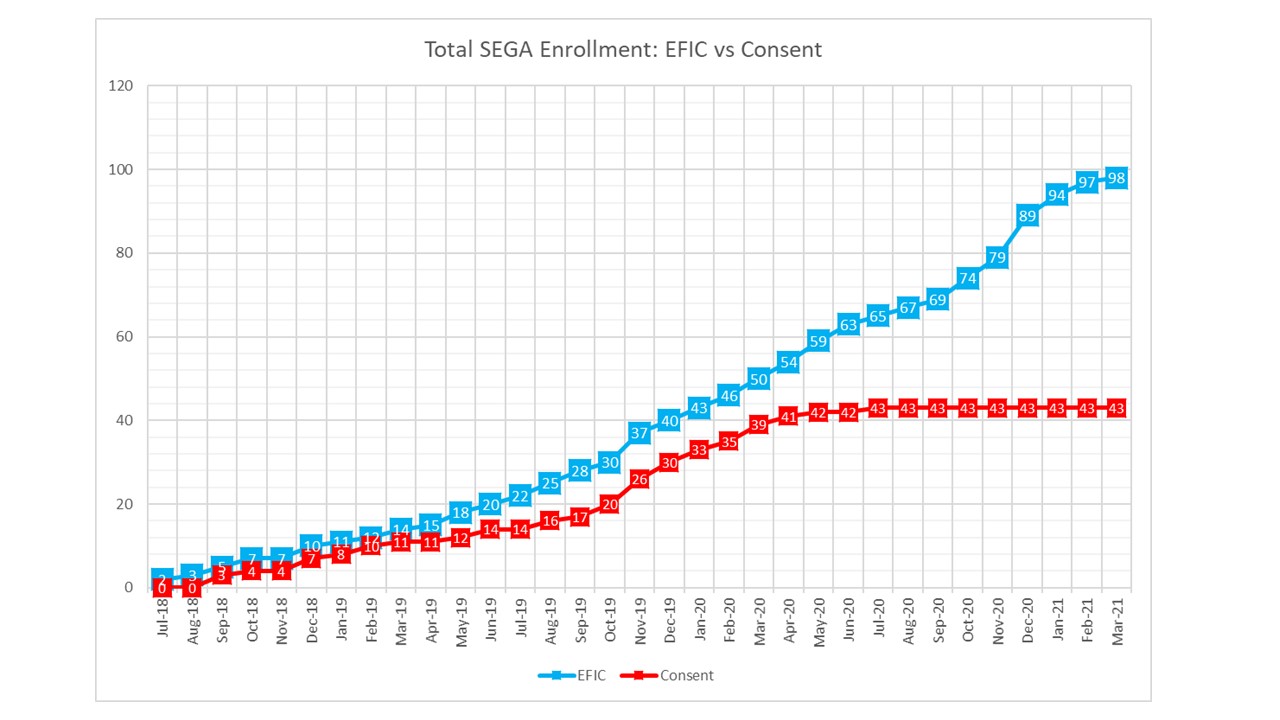
This graph shows the enrollment of subjects where consent was obtained prior to enrollment and randomization (Consent Randomization) or after enrollment and randomization (EFIC Randomization). These numbers are for all SEGA sites, which includes sites with and without an EFIC protocol. Overall, 38.4% of subjects were enrolled without implementing the EFIC protocol.
For more information on how we designed and implemented our novel EFIC approach, please contact SEGA staff here and visit the UTH Clinical Trials Resource Center website regarding the SEGA EFIC process.