Areas of Interest
Research Interests
Desensitization of ß 2- Adrenergic Receptors
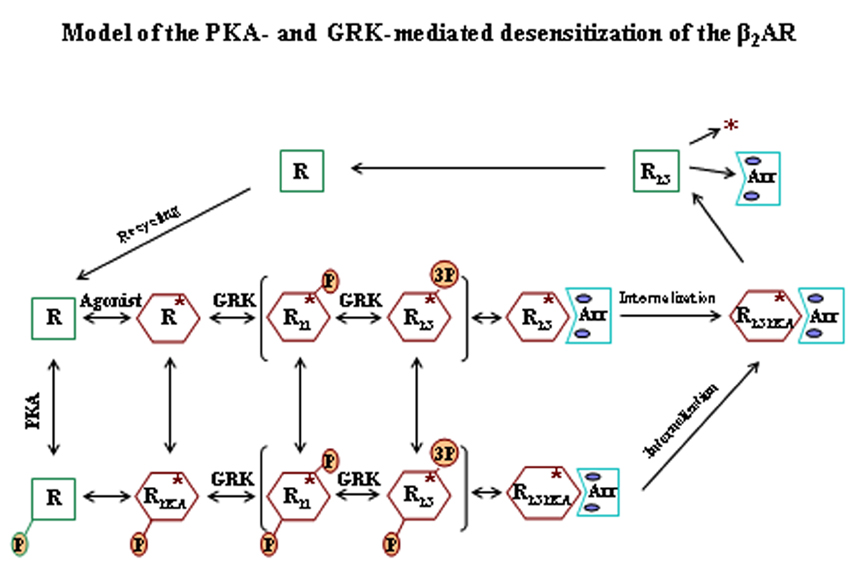
Our group is focused on the mechanisms of activation and desensitization of the human β2-adrenergic receptor (β2AR), and its relevance to various diseases in particular asthma, although drugs that interact primarily with the β2AR are important in numerous other diseases of the cardiovascular and central nervous system. The desensitization of the β2AR in response to agonists is a complex process that involves phosphorylation and regulation by protein kinases, internalization and down regulation. Agonist binding to the receptor triggers the immediate activation and desensitization processes. Our group has been instrumental in defining the precise mechanisms of desensitization, and we have identified the specific receptor sites responsible for the G protein coupled receptor kinase (GRK), cAMP-dependent protein kinase (PKA), and PKC-mediated phosphorylations of the β2AR. GRK phosphorylation leads to arrestin binding and complete uncoupling of the receptor and this in turn causes internalization of the receptor and further desensitization.
A major goal is to define in molecular terms the efficacy of the long-acting β2AR agonists (LABAs) currently in major use in the treatment of asthma such as salmeterol and formoterol. We recently found that salmeterol binding to the receptor, while triggering substantial phosphorylation by GRKs, fails to elicit significant internalization and this has been proposed as a major reason for its efficacy in the treatment of asthma. We are now in the process of developing several approaches to better define the molecular actions of the LABAs that includes: (1) developing, simulating and testing molecular models for the receptor desensitization process, and (2) determining the detailed action of the agonists on gene transcription in human airway smooth muscle cells using microarrays to detect both the overall transcriptome effects and micro-RNA production. several other projects are as follows: (1) defining the specific molecular sites of interactions of the GRKs with the β2AR by site-directed mutagenesis of the GRKs and Evolutionary Trace methodology, (2) developing peptide-based inhibitors of the GRK/receptor interaction through the use of high-throughput screening of both intact cell and cell-free assays, and (4) determination of the specific role played by the family of GRKs (GRKs 2-6) in the desensitization process using various knockdown procedures.
Publications
REFERENCES
- Yin S*, Luo J*, Qian A, Du J, Yang Q, Zhou S, Yu W, Du G, Clark RB, Walters ET, Carlton SM, Hu H. (2013). Retinoids activate the irritant receptor TRPV1 and produce sensory hypersensitivity. J Clin Invest 123(9):3941-51.
- Baameur F, Daniel M, Hui Y, Tran TM, Hammitt RA, Sabui S, McMurray JS, Lichtarge OL, and Clark RB. (2010). Role for the RH Domain of GRK5 and 6 in β2-Adrenergic Receptor and Rhodopsin Phosphorylation, Mol Pharmacol 77, 405-15.
- Vayttaden SJ, Friedman J, Tran TM, Rich TC, Dessauer CW, and Clark RB. (2010). Quantitative Modeling of GRK-Mediated β2AR Regulation. PLoS Computational Biology 6, e1000647.
- Tuan M. Tran, Rasmus Jorgensen, and Richard B. Clark. (2007) Phosphorylation of the β2-Adrenergic Receptor in Plasma Membranes by Intrinsic GRK5. Biochemistry, In review.
- Robert H. Moore, Ellen E. Millman, Veronica Godines, Nicola A. Hanania, Tuan Tran, Hui Peng, Burton F. Dickey, Brian J. Knoll and Richard B. Clark. (2007) Salmeterol stimulation dissociates ß2-adrenergic receptor phosphorylation and internalization. American Journal of Respiratory Cell and Molecular Biology, 36:254-261.
- Tuan M. Tran, Jacqueline Friedman, Faiza Baameur, Brian J. Knoll, Robert H. Moore, and Richard B. Clark. (2007) Characterization of β2- adrenergic receptor dephosphorylation: Comparison with the rate of resensitization. Mol. Pharmacol., 71: 47-60.
- David J. Vaughan, Ellen E. Millman, Veronica Godines, Jacqueline Friedman, Tuan Tran, Wenping Dai, Brian Knoll, Richard B. Clark, and Robert H. Moore. (2006) Role of the GRK-site serine cluster in ß2-adrenergic receptor internalization, desensitization, and ß-arrestin translocation. J. Biological Chemistry, 281:7684-7692.
- Varsha Iyer, Tuan Tran, Jacqueline Friedman, Richard B. Clark, and Brian J. Knoll. (2006) Differential kinetics of agonist-activated phosphorylation and dephosphorylation of the ß2-adrenergic receptor serine 262 and serines 355,356. British Journal of Pharmacology, 147:249-259.
- Tran, T.M., Qunaibi, E., Baameur, F., Moore, R.H., and Clark, R.B. (2004) Characterization of agonist stimulation of PKA- and GRK-site phosphorylation of the β2-adrenergic receptor using phosphoserine-specific antibodies. Mol. Pharmacol., 65:196-206.
