Research
The Proteasome in Aging and Disease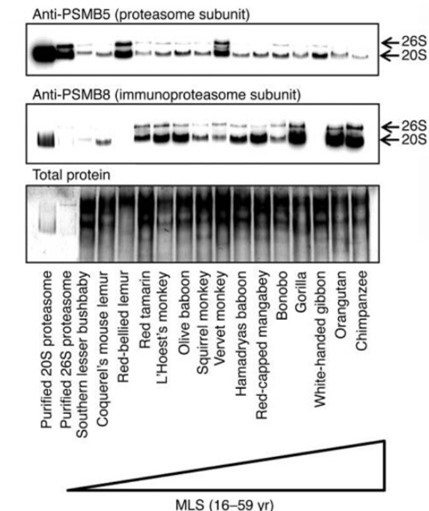
Our research team delves deeply into the proteasome system’s crucial role in aging and the development of age-related diseases. We uncovered that longevity in various species correlates with enhanced proteasome activity, particularly through the immunoproteasome.
Pickering AM, Lehr M, Miller RA. Lifespan of mice and primates correlates with immunoproteasome expression. J Clin Invest. 2015 May;125(5):2059-68. doi: 10.1172/JCI80514. Epub 2015 Apr 13. PubMed PMID: 25866968; PubMed Central PMCID: PMC4463211.
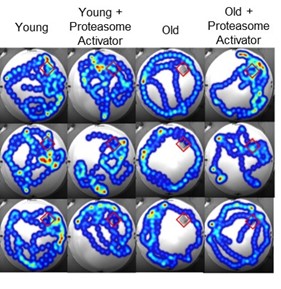 We have found proteasome function to be progressively impaired as a consequence of aging particularly in the brain. Our investigations have shown the selective enhancement of neuronal proteasome activity to be effective in prolonging lifespan and delaying age-related cognitive declines.
We have found proteasome function to be progressively impaired as a consequence of aging particularly in the brain. Our investigations have shown the selective enhancement of neuronal proteasome activity to be effective in prolonging lifespan and delaying age-related cognitive declines.
Parker D, Davidson K, Osmulski PA, Gaczynska M, Pickering AM. Proteasome augmentation mitigates age-related cognitive decline in mice. Aging Cell 2025 <IN PRESS>
Munkácsy E, Chocron ES, Quintanilla L, Gendron CM, Pletcher SD, Pickering AM. Neuronal-specific proteasome augmentation via Prosβ5 overexpression extends lifespan and reduces age-related cognitive decline. Aging Cell. 2019 Oct;18(5):e13005. doi: 10.1111/acel.13005. Epub 2019 Jul 23. PubMed PMID: 31334599; PubMed Central PMCID: PMC6718538.
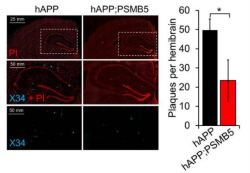 We demonstrate impairment in proteasome function in Alzheimer’s disease and that rescue of this can prevent AD-like deficits. This work paved the way for the development of innovative proteasome agonists which we show to protect against AD-like deficits.
We demonstrate impairment in proteasome function in Alzheimer’s disease and that rescue of this can prevent AD-like deficits. This work paved the way for the development of innovative proteasome agonists which we show to protect against AD-like deficits.
Davidson K, Bano M, Parker D, Osmulski P, Gaczynska M, Pickering AM. β-Amyloid impairs Proteasome structure and function. Proteasome activation mitigates amyloid induced toxicity and cognitive deficits. bioRxiv. 2024 Oct 25;. doi: 10.1101/2024.10.23.619877. PubMed PMID: 39484574; PubMed Central PMCID: PMC11526959.
Chocron ES, Munkácsy E, Kim HS, Karpowicz P, Jiang N, Van Skike CE, DeRosa N, Banh AQ, Palavicini JP, Wityk P, Kalinowski L, Galvan V, Osmulski PA, Jankowska E, Gaczynska M, Pickering AM. Genetic and pharmacologic proteasome augmentation ameliorates Alzheimer’s-like pathology in mouse and fly APP overexpression models. Sci Adv. 2022 Jun 10;8(23):eabk2252. doi: 10.1126/sciadv.abk2252. Epub 2022 Jun 8. PubMed PMID: 35675410; PubMed Central PMCID: PMC9177073.
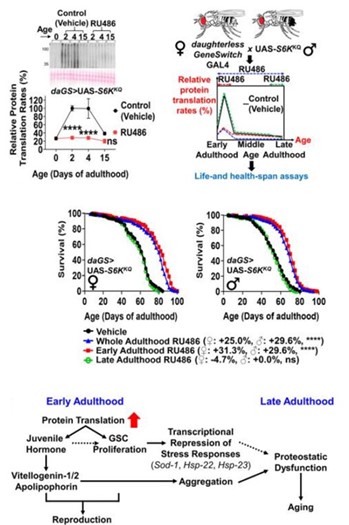 Impacts of early life protein translation on Aging Trajectories
Impacts of early life protein translation on Aging Trajectories
We are interested in understanding how the modulation of protein translation impacts lifespan. We’ve observed that protein translation rates are high in the early stages of life but decline as organisms age. We show that organisms actively repress protein translation in later life mitigating the collapse of proteostasis in later life. Intriguingly, we found that reducing protein translation early in life can significantly increase lifespan, albeit with a trade-off in early-life reproductive capabilities. This phenomenon is linked to a complex interplay of biological processes, including the action of juvenile hormone, the regulation of large lipid transfer proteins, the control of germline stem cell proliferation, and stress response pathways.
Kim HS, Hardiman MM, Pickering AM. Protein translation rates are negatively correlated with lifespan in in-bred Drosophila strains. J Gerontol A Biol Sci Med Sci. 2024 Dec 13;. doi: 10.1093/gerona/glae289. [Epub ahead of print] PubMed PMID: 39670875.
Kim HS, Parker DJ, Hardiman MM, Munkácsy E, Jiang N, Rogers AN, Bai Y, Brent C, Mobley JA, Austad SN, Pickering AM. Early-adulthood spike in protein translation drives aging via juvenile hormone/germline signaling. Nat Commun. 2023 Aug 18;14(1):5021. doi: 10.1038/s41467-023-40618-x. PubMed PMID: 37596266; PubMed Central PMCID: PMC10439225.
Kim HS, Pickering AM. Protein translation paradox: Implications in translational regulation of aging. Front Cell Dev Biol. 2023;11:1129281. doi: 10.3389/fcell.2023.1129281. eCollection 2023. Review. PubMed PMID: 36711035; PubMed Central PMCID: PMC9880214.
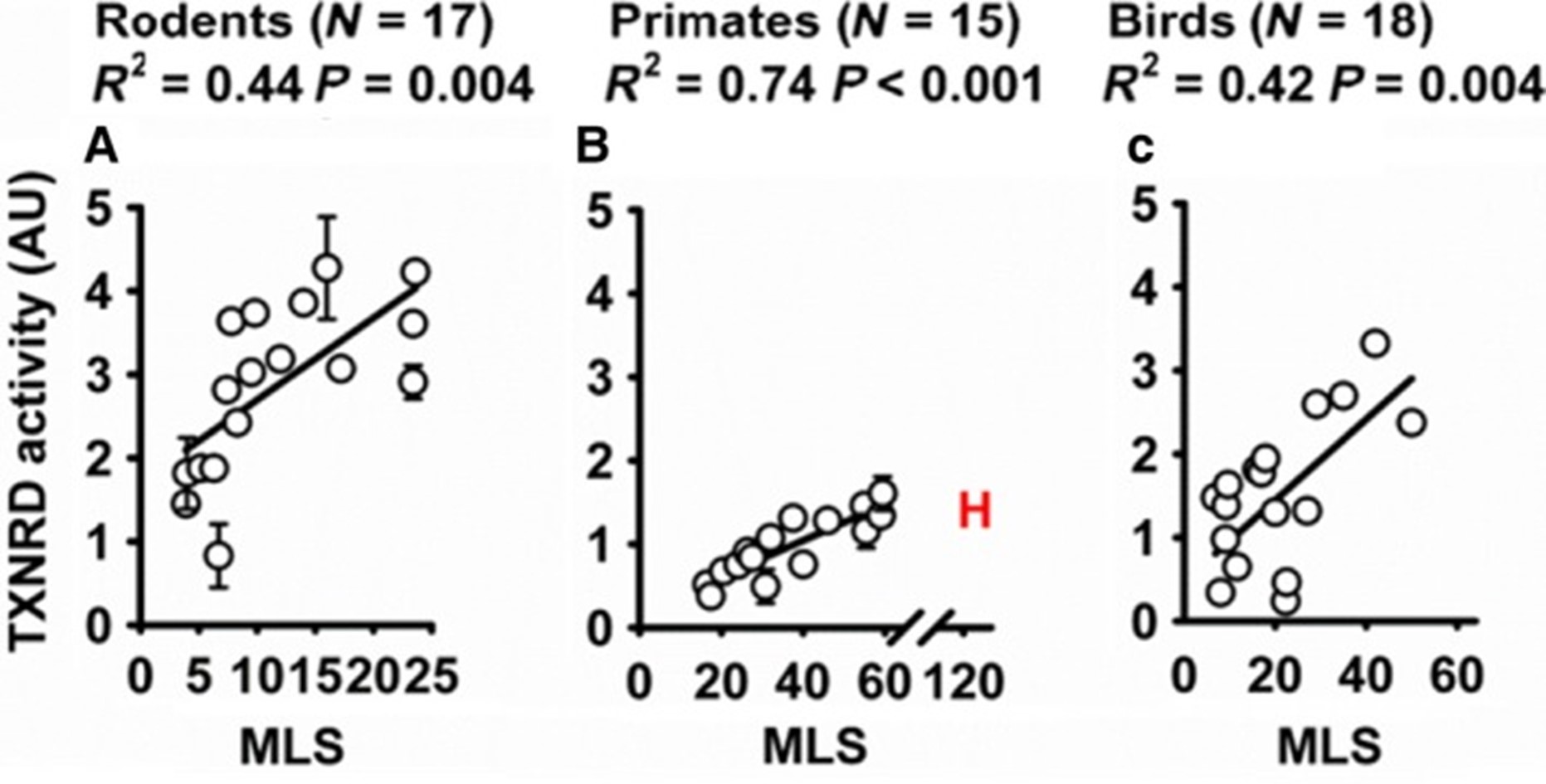 Mitochondrial Thioredoxin Pathway in Aging
Mitochondrial Thioredoxin Pathway in Aging
We are interested in the mitochondrial thioredoxin pathway. We discovered the augmentation of this system in long-lived species, ranging from rodents and primates to birds. This interest led us to identify significant upregulation of the mitochondrial thioredoxin system in these species, suggesting a powerful link between thioredoxin activity and longevity. Further investigations revealed that this system not only plays a crucial role in slowing aging in mouse models but also enhances lifespan and vitality in the fruit fly Drosophila melanogaster when the enzyme is augmented.
Pickering AM, Lehr M, Gendron CM, Pletcher SD, Miller RA. Mitochondrial thioredoxin reductase 2 is elevated in long-lived primate as well as rodent species and extends fly mean lifespan. Aging Cell. 2017 Aug;16(4):683-692. doi: 10.1111/acel.12596. Epub 2017 May 5. PubMed PMID: 28474396; PubMed Central PMCID: PMC5506402.
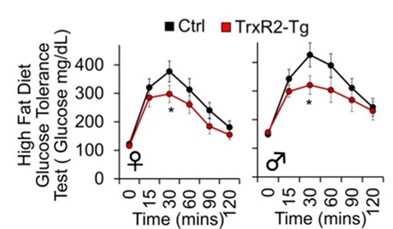 Moreover, our research has unveiled the mitochondrial thioredoxin system’s capacity as an innovative metabolic regulator, offering protection against metabolic diseases.
Moreover, our research has unveiled the mitochondrial thioredoxin system’s capacity as an innovative metabolic regulator, offering protection against metabolic diseases.
Chocron ES, Mdaki K, Jiang N, Cropper J, Pickering AM. Mitochondrial TrxR2 regulates metabolism and protects from metabolic disease through enhanced TCA and ETC function. Commun Biol. 2022 May 16;5(1):467. doi: 10.1038/s42003-022-03405-w. PubMed PMID: 35577894; PubMed Central PMCID: PMC9110405.