Research
The Neuroimmune Interaction Laboratory mainly focuses on the role of microglia, the highly dynamic immune cells in the central nervous system, in clinically relevant pathologies such as epilepsy, pain, stroke and autoimmune diseases.
The primary aim of the lab is to understand microglia-neuron communication in normal and diseased brain. Particularly, the team investigates the molecular mechanisms underlying microglia sensing and regulation of neuronal activities.
With the use of advanced genetic models along with a combination of two-photon imaging, electrophysiology, electroencephalography, molecular biology techniques and awake-behavioral studies in rodent models, the team reveals microglia-specific receptors and ion channels in microglial function. The long-term vision of the Wu lab research is to identify and manipulate microglia-specific therapeutic targets that will complement existing strategies for the treatment of brain diseases, including epilepsy, pain, stroke, and autoimmune diseases.
Questions:
1, What is the physiological role of microglia in the central nervous system?
2, What signals mediate microglia-neuron communication and how do microglia integrate into neuronal circuits?
3, What are the molecular mechanisms and functional consequences of microglia activation in brain diseases?
Approaches:
The Neuroimmune Interaction Laboratory employs genetic animal models along with a combination of advanced tools such as two-photon imaging, electrophysiology, electroencephalography, molecular biology techniques and awake-behavioral studies in rodent models.
Ongoing Projects:
|
1. Microglial dynamics and chemotaxis |
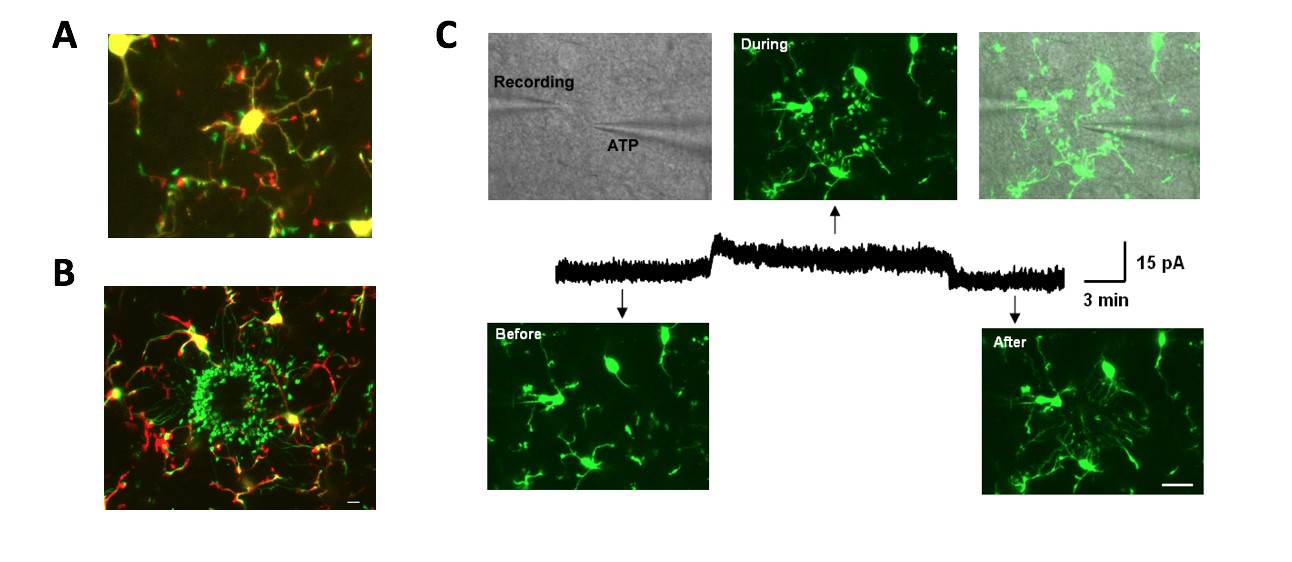 Microglial imaging/elctrophysiology to study microglial dynamics and chemotaxis
Microglial imaging/elctrophysiology to study microglial dynamics and chemotaxis
A, Time-lapse overlay images at 0 min (red) and at 9 min (green) showed the extension or retraction of microglial in a single microglia.
B, Local puff application oprocesses f ATP induced chemotaxis of microglial processes towards ATP. The merged picture is the overlay of imaging at 0 min (red) and 30 min (green).
C, ATP-induced microglial chemotaxis (upper) requires K channel activation (middle). Scale bar, 15 mm.
| 2. Microglial ion channel/receptor electrophysiology |
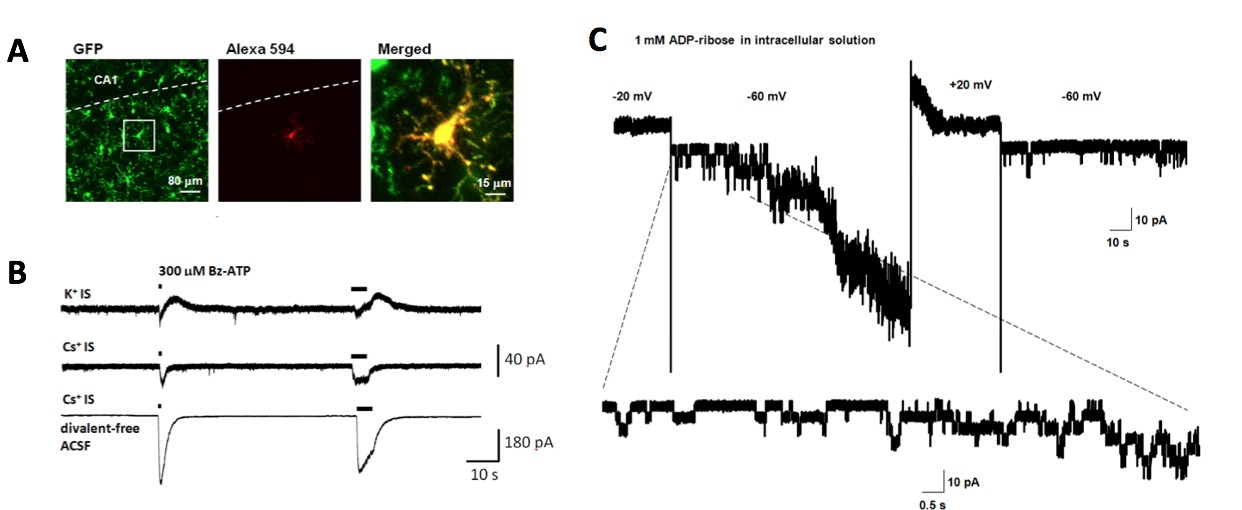
Microglia whole-cell recordings to study ion channels and receptors
A, Microglia is visualized with GFP fluorescence and labeled by a red dye through patch clamp recording pipette.
B, P2X7 ATP receptor-mediated currents observed in brain microglia. Bz-ATP is a P2X7 receptor agonist.
C, TRPM2-mediated currents observed in brain microglia. ADP-ribose is a TRPM2 channel agonist.
| 3. Microglia-neuron physical interactions |
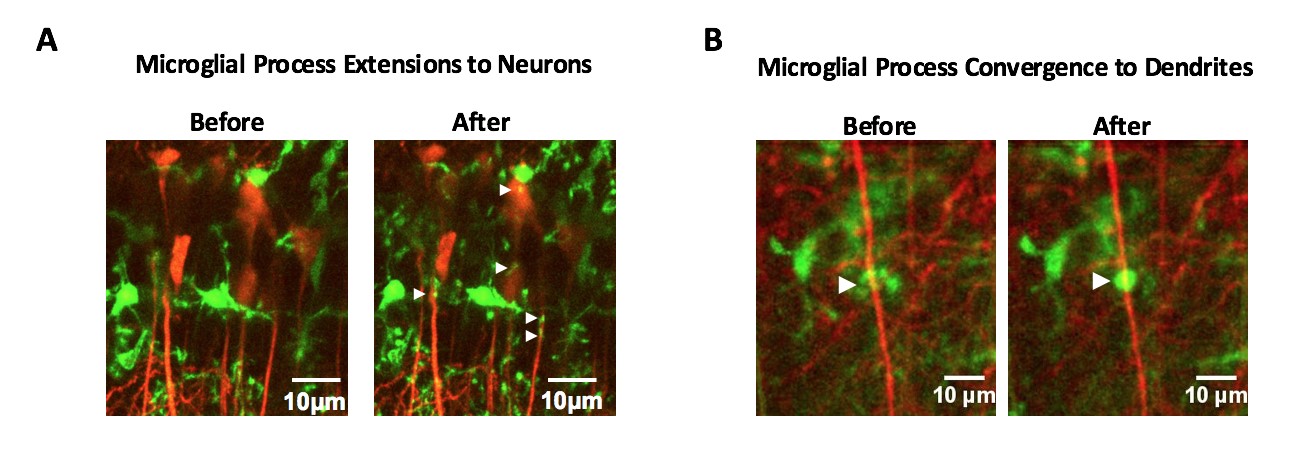
Two-photon live imaging of microglia-neuron interaction in brain slices or in vivo
A, Microglial process extension triggered by glutamate treatment. Microglia (green) extend their dynamic processes to interact with neuronal (red) cell body and dendrites (arrowheads).
B, Microglia process convergence is where microglial processes converge at a hotspot (arrowheads) on a neuronal dendrite after lowering extracellular Ca2+ or under experimental seizures in mice.
| 4. Microglia in neurodegeneration |
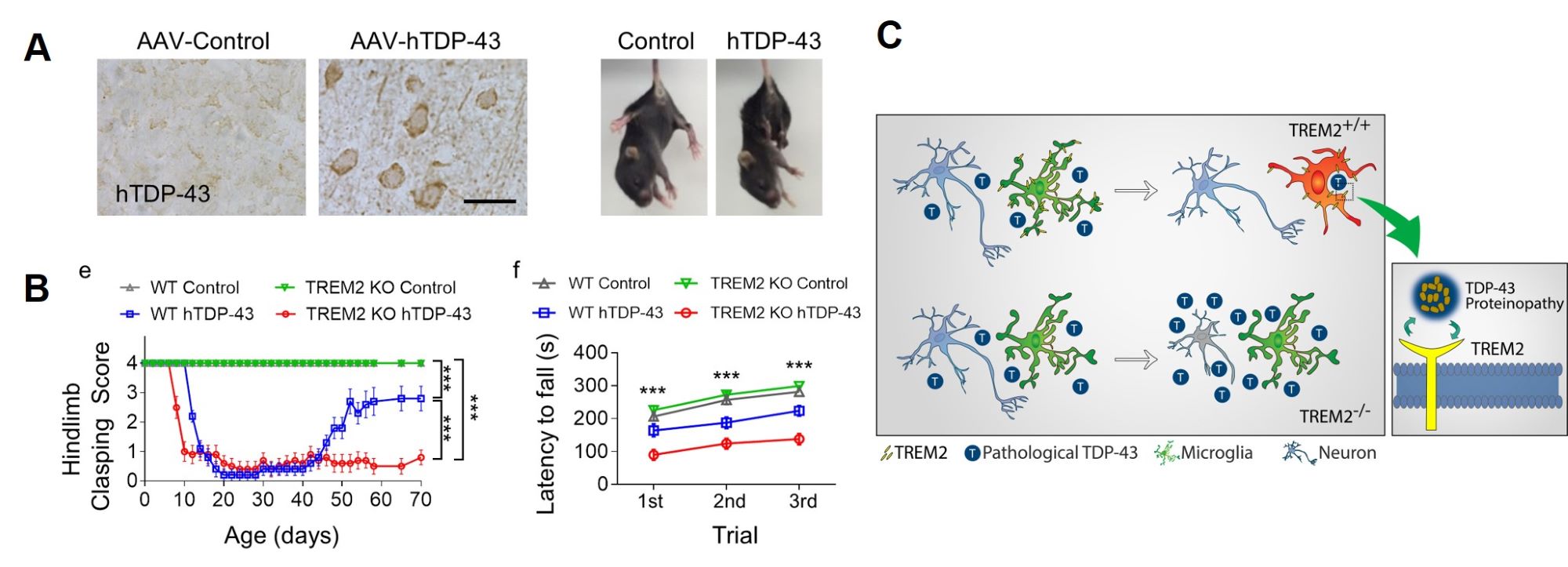
Microglial TREM2 in the protection against TDP-43-related neurodegeneration
A, TDP-43 neurodegeneration in a mouse model induced by ICV injection of AAV-hTDP-43.
B, Microglial TREM2 deficiency aggravates hTDP-43-induced behavioral deficits.
C, TREM2 mediates neuroprotective effects of microglia by sensing and phagocytosing extracellular TDP-43 and degenerating neurons.
| 5. Microglia in epilepsy |
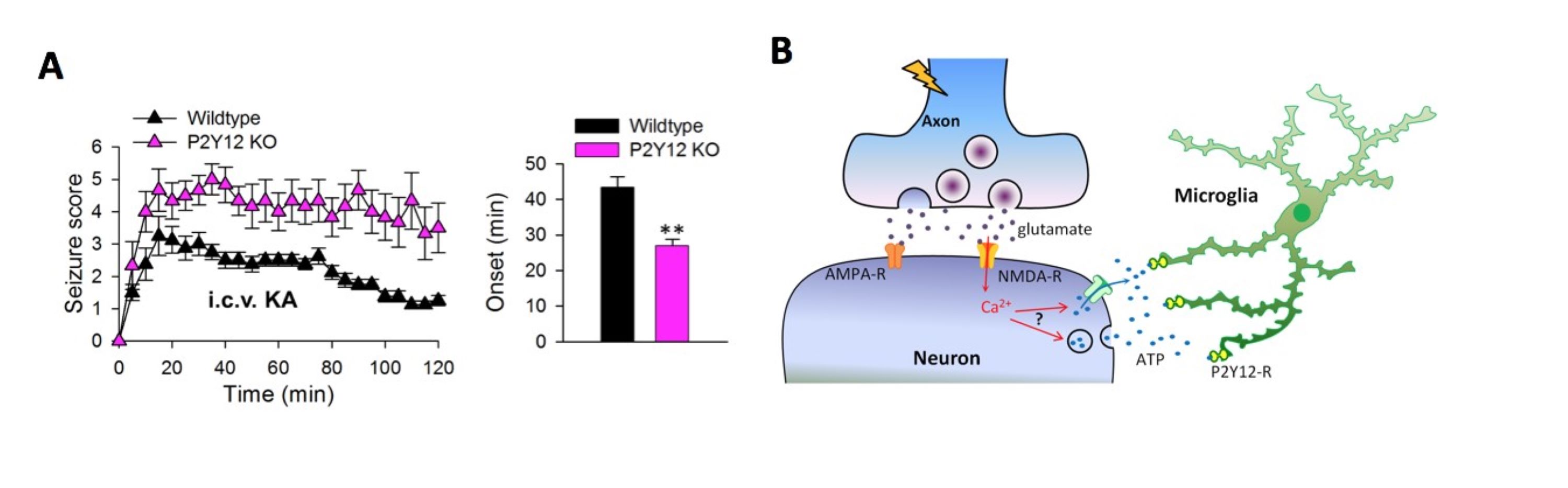
Microglia P2Y12 receptors modulate experimental epileptic phenotypes
A, Genetic ablation of P2Y12 receptors in microglia increased seizure phenotypes induced by Kainic Acid (KA) treatment including more severe behavioral seizures (left) and an earlier onset of the seizures (right).
B, The diagram showing increased glutamate activates NMDA receptors that triggers ATP release to elicit microglial attraction through its P2Y12 receptors under seizure conditions.
| 6. Microglia in pain |
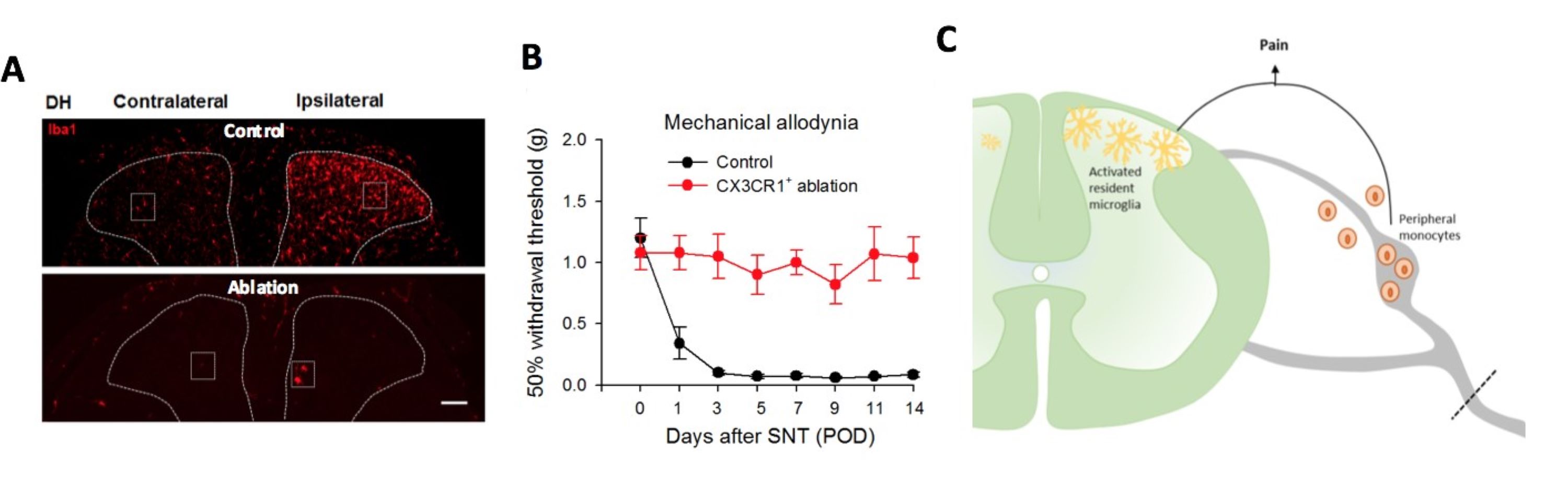
Microglia and monoctyes promote neuropathic pain
A, Neuropathic pain increases microglial reactivity in the ipsilateral dorsal horn of the spinal cord (top) while targeted genetic ablation of microglia successfully eliminated them.
B, Mechanical pain phenotypes were abolished in mice after microglia/monocyte ablation.
C, Schematic summary indicating contributions of both resident microglia and peripheral monocytes to the progression of neuropathic pain.