Areas of Interest
Research Interests
Regulatory Mechanisms and (Patho) physiological Functions of Mammalian Circadian Clocks
In response to daily environmental changes imposed by Earth’s rotation, almost all species, ranging from cyanobacteria to humans, have evolved physiological and behavioral rhythms, called circadian rhythms. Circadian rhythms are not passive responses to environmental changes; rather, they are driven by an active clock system, capable of anticipating changes and coordinating tissue specific function and generating systemic output responses. The harmony between our intrinsic biological timing and the daily environmental oscillation is critical to physiological well-being; conversely, disrupted circadian rhythms have been shown to cause or increase the risk of various chronic diseases. In our lab, we focus on delineating fundamental cellular mechanisms in circadian rhythms and also deciphering physiological and pathological roles of the clock. Our long-term goal is to translate such fundamental mechanistic knowledge into new drug targets and therapeutic strategies for improved prevention and treatment of chronic diseases.
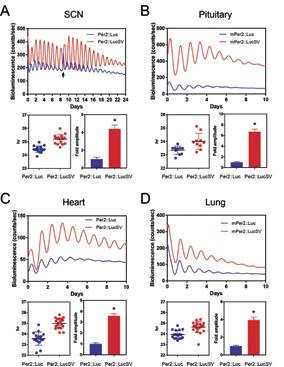
Figure 1. Comparison of real-time bioluminescence analysis of circadian expression of Per2::Luc and Per2::LucSV. Left panels: representative bioluminescence traces. Right panels: periods from individual replicates.
Currently, our lab are pursuing several projects, using an integrative approach combining mouse models with molecular and cellular mechanistic studies. In one project, we investigate the role of miRNAs in circadian clock regulation using a second-generation circadian reporter mouse line Per2::LucSV (Figure 1), with a particular focus on the mechanistic relationship between clock robustness and metabolic health. In another project, we investigate the role of a circadian E3 ligase in heart failure induced skeletal muscle atrophy using a novel mouse model. Finally, we continue to pursue cloning of novel circadian clock mutants recently identified by a recessive mouse genetic screen.
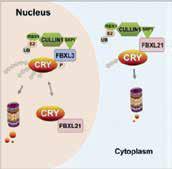
Figure 2. Circadian clock periodicity is governed by two paralogous F-box proteins FBXL3 and FBXL21. These two E3 ligases regulate levels of the circadian components CRY through a balance of protein degradation and stabilization. FBXL21 plays a dual role: protecting CRY from FBXL3 degradation in the nucleus and promoting CRY degradation within the cytoplasm. (Yoo et al., Cell 2013).
Selected Publications
Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. 2004. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 101(15):5339-46.
Yoo SH, Mohawk JA, Siepka SM, Shan Y, Huh SK, Hong HK, Kornblum I, Kumar V, Koike N, Xu M, Nussbaum J, Liu X, Chen Z, Chen ZJ, Green CB, Takahashi JS. 2013. Competing E3 ubiquitin ligases govern circadian periodicity by degradation of CRY in nucleus and cytoplasm. Cell. 152(5):1091-1105.
He B, Nohara K, Park N, Park YS, Guillory B, Zhao Z, Garcia JM, Koike N, Lee CC, Takahashi JS, Yoo SH, Chen Z. 2016. The small molecule Nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metabolism. 23: 610-21.
Education and Training
BS
Biological Sciences, Ehwa Women’s University
MS
Biological Sciences, Korea Advanced Institutes of Science and Technology
PhD
Biological Sciences, Korea Advanced Institutes of Science and Technology
Postdoctoral Fellow
Florida State University and Northwestern University
Graduate Program Affiliations
Molecular and Translational Biology
