Areas of Interest
Research Interests
Folding and Assembly of Membrane Proteins
Lipid and Protein Topogenesis in Bacterial and Cancer Cells
Do Membranes Tell Proteins How to Fold?
Biological membranes manifest a myriad of unique phospholipid molecules and an amazing diverse array of surfaces and forces (electrostatic, elastic, hydrophobic, electrophoretic) that can fold and orient an integral membrane protein. By taking advantage of the simple yet well-characterized Escherichia coli bacterial system, we can easily manipulate either temporally or in a dose-dependent manner the lipid composition in genetically altered strains, lipid-based solid supports, mixed lipid-detergent micelles, or lipid bilayers of liposomes. These systems coupled with in vitro membrane protein refolding, reconstitution techniques and membrane protein topology assays are being used to establish the function of individual lipids in such complex processes as insertion of proteins in to the lipid bilayer and folding and topogenesis of membrane proteins. Given the similarity between in vivo and in vivo results, the rules governing lipid-protein interactions are easily extrapolated to folding and assembly rules that are applicable to more complex biological systems.
Lipid-assisted Membrane Protein Folding: Lipids as Molecular Chaperones (Lipochaperones)
Traditionally, molecular chaperones have been classified as proteins that bind transiently to substrate proteins to promote their proper folding by interacting non-covalently and transiently with non-native folding intermediates and not with either the native or totally unfolded protein. We developed novel techniques (Eastern-Western blotting technique, coupled cell-free protein and phospholipid biosynthesis systems, advanced substituted-cysteine accessibility method as applied to transmembrane domains (SCAMTM)) to analyze the effects of lipids on membrane structure and function. Using these techniques coupled with genetic manipulation of E. coli “lipid” genes led us to discover a novel function for membrane phospholipids acting as molecular chaperones for (lipochaperones) and topological determinants of membrane proteins during initial insertion and folding or after complete folding into a native structure. Eastern-Western blotting was invented as the first technique, which allowed the identification of transient effects of lipids on membrane protein conformation.
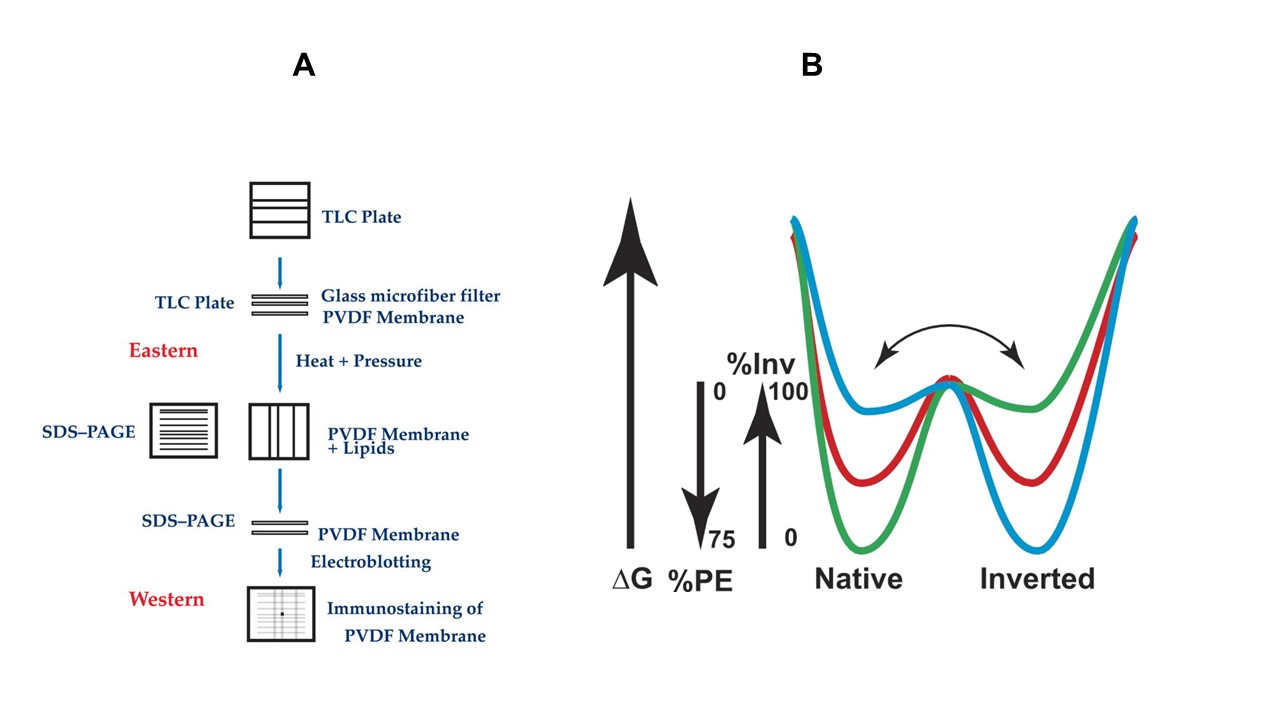
A. Eastern-Western blotting technique makes possible the detection of proper membrane protein refolding after transfer of proteins onto a nitrocellulose membrane pre-blotted with selected phospholipids.
B. Funnel-like intermolecular energy landscape for lipid-protein interactions during lipid-assisted protein folding. Dual minima energy folding funnel for LacY as a function of membrane lipid composition.
- Bogdanov M, Sun J, Kaback HR and Dowhan W: A phospholipid acts as a chaperone in assembly of membrane transport protein. J. Biol. Chem. 271: 11615-11618, 1996.
- Bogdanov M and Dowhan W: Phospholipid-assisted protein folding: phosphatidylethanolamine is required at a late step of the conformational maturation of the polytopic membrane protein lactose permease. EMBO J. 17: 5255-5264, 1998.
- Bogdanov M., Umeda M and Dowhan W: Phospholipid-assisted refolding of an integral membrane protein. Minimum structural features for phosphatidylethanolamine to act as a molecular chaperone. J. Biol. Chem. 274: 12339-12345, 1999.
- Bogdanov M and Dowhan W: Lipid-assisted protein folding J. Biol. Chem. 274: 36827-36830, 1999.
- Bogdanov M, Heacock P, Guan Z and Dowhan W: Plasticity of lipid-protein interactions in the function and topogenesis of the membrane protein lactose permease from Escherichia coli. Proc. Natl. Acad. Sci. USA. 107(34):15057-62, 2010.
- Dowhan W and Bogdanov, M: Molecular genetic and biochemical approaches for defining lipid-dependent membrane protein folding. Biochim. Biophys. Acta. 1818(4):1097-107, 2012.
Experimental questions we focus on
- How do phospholipids facilitate membrane protein folding?
- What structural features of a lipid account for its ability to act as a molecular chaperone?
- What is the minimum structural feature of a membrane protein folded with phospholipid assistance?
- Do lipids act as lipochaperones promiscuously: e.g. show broad specificity for binding non-native proteins or specifically involved in the folding of only very limited number of substrate proteins?
- How do protein and non-protein molecular chaperones cooperate in vivo?
- How widely might phospholipid-assistance apply to membrane protein folding?
- How do healthy protein commit “deadly” misfolding and self-aggregation?
- Do phospholipids act as molecular anti-chaperones?
Lipid-dependent membrane protein topogenesis: lipids as topological determinants
Studying membrane protein topogenesis focuses on understanding and predicting how a given protein sequence will fold and orient itself in a given phospholipid environment.
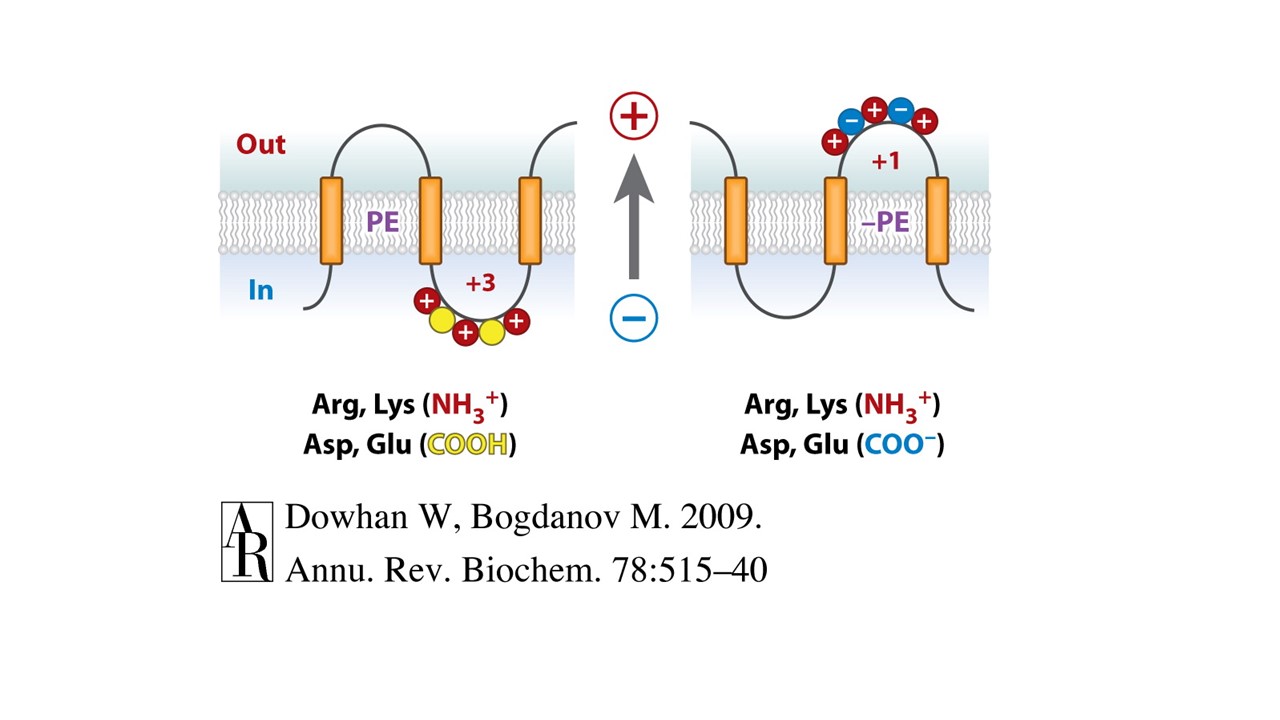
Reagent strains of Escherichia coli cells in which a particular lipid can be either eliminated, substituted by “foreign lipids”, or its level controlled temporally during membrane protein synthesis were developed to assess how membrane lipid composition affects protein topogenesis. Advanced methods for determining protein topology (in particular substituted monocysteine accessibility method (SCAMTM) were developed to analyze protein transmembrane topological organization as a function of the lipid environment in whole cells, inside out and right side out membrane vesicles and liposomes. We have used the well-characterized lactose permease (LacY) and several other secondary transporters of E. coli to establish the role of the major phospholipid (phosphatidylethanolamine, PE) in determining membrane protein topological organization. We established for the first time that membrane lipid composition is a topology determinant of a polytopic membrane protein at the time of initial membrane insertion and assembly. Furthermore, protein topological organization can change and is responsive to changes in the lipid environment after initial folding. These results clearly demonstrated that the lipid composition is a determinant of transmembrane domain orientation and challenges the dogma that once a transmembrane domain is membrane inserted and oriented during assembly it is static and not subject to change. Common dogma dictates a high thermodynamic barrier to transmembrane domain flipping after insertion and predicts that flip-flop of integral proteins does not occur. However post-translational and even post-insertional reorientation of membrane proteins is not unprecedented. Such flip-flopping proteins are synthesized in one direction and then flipped following synthesis of the remainder of the protein or even after the insertion is fully completed. Lipid-protein interactions (particularly net charge neutral phospholipids such as PE) appear to affect the potency of charged residues as topological signals and therefore drive membrane protein topogenesis according Positive-Inside Rule by damping the translocation potential of negative residues in favor of the cytoplasmic retention potential of positive residues (Charge-Balance Rule).
Selected References
- Bogdanov M, Heacock, P and Dowhan, W: A polytopic membrane protein displays a reversible topology dependent on membrane lipid composition. EMBO J. 21: 2107-2116, 2002.
- Wang X, Bogdanov M and Dowhan W: Topology of polytopic membrane protein subdomains is dictated by membrane phospholipid composition. EMBO J. 21: 5673-5681, 2002.
- Bogdanov M, Zhang W, Xie J and Dowhan W: Transmembrane protein topology mapping by the substituted cysteine accessibility method (SCAMTM): application to lipid-specific membrane protein topogenesis. Methods. 36:148-171, 2005.
- Bogdanov M, Xie J, Heacock P and Dowhan, W: To flip or not to flip: protein–lipid charge interactions are a determinant of final membrane protein topology J. Cell Biology. 182: 925-935, 2008.
- Bogdanov M, Xie J and Dowhan W: Lipid-protein interactions drive membrane protein topogenesis in accordance with the positive-inside rule. J. Biol. Chem. 284: 9637-9641, 2009.
- Dowhan W and Bogdanov M: Lipid-dependent topogenesis. Annu. Rev. Biochem. 78: 515-540, 2009.
- Bogdanov M and Dowhan W: Lipid-dependent generation of dual topology for a membrane protein. J. Biol. Chem. 287: 37939-37948, 2012.
- Vitrac H, Bogdanov M, Dowhan W: In vitro reconstitution of lipid-dependent dual topology and postassembly topological switching of a membrane protein. Proc Natl Acad Sci USA. 110(23):9338-9343, 2013.
- Bogdanov M, Dowhan W, Vitrac H: Lipids and topological rules governing membrane protein assembly. Biochim Biophys Acta. 1843(8): 1475-1488, 2014.
- Bogdanov, M. Mapping of Membrane Protein Topology by Substituted Cysteine Accessibility Method (SCAMTM) Methods in Molecular Biology. 1615:105-128, 2017.
- Bogdanov, M., Vitrac. H., Dowhan, W. Flip-flopping Membrane Proteins: How the Charge Balance Rule Governs Dynamic Membrane Protein Topology. Biogenesis of Fatty Acids, Lipids and Membranes (Geiger, O., Ed) Second Edition, Elsevier Press, Amsterdam, 2018.
Experimental questions we focus on
- Where, how and when do membrane proteins make their topological “decision”?
- Are topogenic signals recognized and “decoded” by given a lipid profile or “interpreted” by the insertion machinery?
- How do individual lipids and components of the translocon and insertase contribute to membrane protein insertion?
- Transmembrane protein topology: static or dynamic?
- Is the lipid bilayer really a non-flipping zone for integral membrane proteins?
- How to overcome the energy cost of the flip-flop movement of a large hydrophilic domain across the membrane?
- Can lipids manipulate a single membrane protein substrate to achieve multiple topological forms?
- How are transmembrane protein topological inversions governed and controlled: thermodynamically or kinetically?
- Do β-barrel membrane proteins follow the Positive-Outside rule?
- Whether topological differences originate co-translationally during membrane insertion or are they induced by changes in lipid composition as eukaryotic proteins move through different organelles to their final destination?
- Do lipid dependent topological disorders exist?
- Is evolutionary pressure required to maintain a delicate balance between membrane protein structural features and lipid environment for proper function and topological maturation?
Role of Gram-negative bacterial envelope remodeling in the development of resistance to antibiotics, intracellular survival and ability to resist innate immunity of the host
The goal of my third current project is to test a hypothesis that conformation of outer membrane b-barrel porins from pathogenic bacteria may adapt to in vivo changes in phospholipid composition in order to optimize or modulate their activity or folding and assembly pathway. The goal of this project is to test a hypothesis that specific lipids (cold and shock lipids in this case) can optimize protein function and conformation and promote proper folding at low or elevated temperatures i.e. act in the way molecular chaperones of protein origin do. Specifically, we are addressing the question whether these adaptive changes are important not only for poring-mediated antibiotic permeability but also for the development of bacterial virulence and resistance to antibiotics and innate immune system of infected host and thus could be utilized to increase drug susceptibility of pathogenic microorganisms to known antibiotics and innate immune system.
Selected References
- Zheng, L., Lin, Y., Lu, S, Zhang, J, Bogdanov M. Biogenesis, transport and remodeling of lysophospholipids in Gram-negative bacteria. Biochim Biophys Acta. S1388-1981(16) 30332-8, 2016.
- Sanina, N., Pomazenkova, L., Bakholdina, S., Chopenko, N., Zabolotnaya, A., Reutov, V., Stenkova, A., Bystritskaya, E., and Bogdanov, M. Relationship between adaptive changing of lysophosphatidylehanolamine content in bacterial envelope and ampicillin sensitivity of Yersinia pseudotuberculosis. Journal of Molecular Microbiology and Biotechnology, 28 (5) 236-239, 2019.
My current interests include also “lipid” genes interchangeability in bacteria, physiological significance of bacterial “lipid” genes multiplicity, the origin and maintenance of transmembrane phospholipid asymmetry, physiological role of lysolipids in bacterial and mammalian membranes, membrane protein and lipid topogenesis in normal and cancer cells.
Education and Training
MSc
Biochemistry, Voronezh State and Moscow State Universities
PhD
Biochemistry and Physiology of Microorganisms, USSR Academy of Sciences
Postdoctoral Fellow
Biochemistry and Molecular Biology, UTHealth McGovern Medical School
Graduate Program Affiliations
Molecular and Translational Biology
Microbiology and Infectious Diseases
