
Harry Karmouty-Quintana, PhD
- Associate Professor
- Director, Graduate Program in Biochemistry and Cell Biology
- Director, Pulmonary Center of Excellence
Areas of Interest
Research Interests
Mechanisms of pulmonary hypertension (PH) associated with chronic lung diseases such as chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF); Mechanisms of adenosine signaling and hyaluronan that lead to PH in chronic lung diseases.
Novel Mechanisms in Chronic Lung Disease
Chronic Lung Diseases (CLDs) such as Idiopathic Pulmonary Fibrosis (IPF), Chronic Obstructive Pulmonary Disease (COPD) and Pulmonary Hypertension (PH) represent the third-leading cause of death in the US. Despite reductions in mortality in cancer and cardiovascular disease, mortality rates for CLD have remained unaffected over the last decade. This a direct result of disparate funding allocated toward research in CLD that in no way reflects the heavy societal burden of the disease, as well as an overwhelming lack of effective therapies capable of targeting the airspace and vascular remodeling that is a pervasive hallmark in many CLDs. Through the use of experimental models of disease and access to the UTHealth Pulmonary Center of Excellence Bio-bank, our research aims to understand the processes that govern disease progression in order to develop new therapies that are desperately needed.
Understanding How Changes in the Lung Extracellular Matrix (ECM) Lead to Vascular Remodeling in PH Associated with IPF
The presence of PH in patients with IPF is the single most significant predictor of mortality, yet there are no cures at present that effectively prevent or treat PH in patients with IPF. In this project we have identified increased levels of hyaluronan, a component of the lung ECM, in the lung of patients with a diagnosis of PH associated with IPF. We next demonstrate that the increase in hyaluronan is mediated by an elevation in hyaluronan synthase 2 (HAS2), an enzyme that makes hyaluronan. Finally, we demonstrate that treatment with hymecromone, a drug that inhibits HAS2, is able to reduce levels of hyaluronan and treats PH associated with IPF.
We are currently examining the mechanisms that lead to HAS2 increases in patients with IPF and examining how selective deletion of HAS2 from smooth muscle cells contributes to the development of PH.
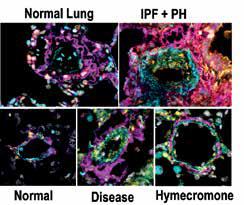
Alpha Smooth muscle actin (cyan), hyaluronan (magenta), hyaluronan binding protein2 (HAPB2, yellow) and DAPI (grey) from human lungs (top panels) or from mice models of chronic lung disease (bottom panels).
Evaluating How Changes in MRNA 3’UTR Length Participates in the Development of COPD
In collaboration with leaders in RNA-biology and with Dr. Leng Han, an expert in bio-informatics at UTHealth, we have identified changes in the 3’UTR length of mRNAs in patients with COPD. These mRNAs encode many proteins associated with the development of disease. In addition, we show that a protein known as cleavage factor 25 (CFIm25) regulates the length of the 3’UTRs and that depletion of this protein results in global shortening of mRNAs. This protein was also found to be depleted in patients with COPD. Our research efforts are aimed at elucidating how 3’UTR shortening contributes to the development of COPD, using tissue samples from the UTHealth Pulmonary Center of Excellence bio-bank and sophisticated experimental models of disease.
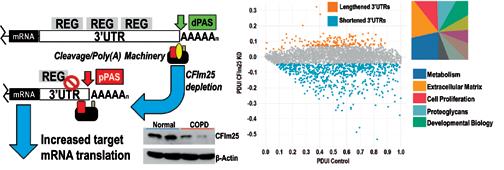
Alternative polyadenylation (APA) and subsequent 3’UTR shortening consistent with reduced CFIm25 in COPD tissue (Left panel). Percentage of distal PAS usage index (PDUI) showing shortened transcripts and pathway analysis (right panel).
Selected Publications
Collum SD, Chen NY, Hernandez AM, Hanmandlu A, Sweeney H, Mertens TCJ, Weng T, Luo F, Molina JG, Davies J, Horan IP, Morrell NW, Amione-Guerra J, Al-Jabbari O, Youker K, Sun W, Rajadas J, Bollyky PL, Akkanti BH, Jyothula S, Sinha N, Guha A, Karmouty-Quintana H. 2017. Inhibition of Hyaluronan Synthesis Attenuates Pulmonary Hypertension Associated with Lung Fibrosis. Br J Pharmacol. 174:3284-3301.
Garcia-Morales LJ, Chen NY, Weng T, Luo F, Davies J, Philip K, Volcik K, Melicoff E, Amione-Guerra J, Bunge RR, Bruckner BA, Loebe M, Eltzschig HK, Pandit LM, Blackburn MR, Karmouty-Quintana H. 2016. Altered Hypoxic-Adenosine Axis and Metabolism in Group III Pulmonary Hypertension. Am J Respir Cell Mol Biol. 54(4):574-83.
Karmouty-Quintana H, Weng T, Garcia-Morales LJ, Chen NY, Pedroza M, Zhong H, Molina JG, Bunge R, Bruckner BA, Xia Y, Johnston RA, Loebe M, Zeng D, Seethamraju H, Belardinelli L, Blackburn MR. 2013. Adenosine A2B receptor and hyaluronan modulate pulmonary hypertension associated with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 49(6):1038-47.
Education and Training
BS
Pharmacology, King’s College
PhD
Pharmacology and Biophysics, King’s College
Postdoctoral Fellow
McGill University and UTHealth McGovern Medical School
Graduate Program Affiliations
Molecular and Translational Biology
Biography
I previously trained in Montreal, Quebec at McGill University from 2006 to 2010 where I completed my first postdoc on airway smooth muscle remodeling in asthma. Prior to that I lived in Basel, Switzerland, where I pursued my PhD on “Experimental Pulmonary Diseases assessed by Magnetic Resonance Imaging”, a joint venture with Novartis and King’s College London (UK) where I graduated in 2006. Having spent a lot of time in cold countries, I was happy to move to Houston in May 2010 where I started as a postdoc and was promoted to Research Assistant Professor in September 2012. Other than lung diseases, I am interested in ocean life; I have a saltwater aquarium at home and I am a certified scuba diver! Originally from Barcelona, I may be hard to find if FC Barcelona is playing a champions league game or against Real Madrid!