Team Serysheva
Our research aims to understand molecular mechanisms underlying transport of molecules into and out of the cell across the surface membrane, or between different intracellular compartments through structure-functional studies of integral membrane proteins known as ion channels, and the macromolecular complexes they form. Our specific emphasis is on structural and functional characterization of calcium channels that mediate the transfer of Ca2+ ions through the hydrophobic bilayer of the cell membrane. Ca2+ ions are ubiquitous and versatile signaling molecules that serve many diverse cellular functions ranging from gene transcription to secretion, from proliferation to cell death. The knowledge about the three dimensional (3D) architecture of ion channels is required to understand molecular basis of channel gating (opening/closing process), and how this process is controlled by a wide variety of endogenous molecules and pharmacological modifies. To answer these questions we use a combination of electron microscopy and computer reconstruction techniques in conjunction with biochemical, electrophysiological and molecular biological approaches. Our structure research efforts include: 1) purification of ion channels from natural sources or from high-level expression systems; 2) electron cryomicroscopy (cryo-EM) of the purified channel assemblies and cellular organelles; 3) computer image processing and 3D reconstruction; 4) structure analysis and annotation using combination of visualization and computational tools; 5) prediction of functional roles of the identified structural domains via bioinformatics; 6) functional validation and discovery of molecular mechanisms of action.
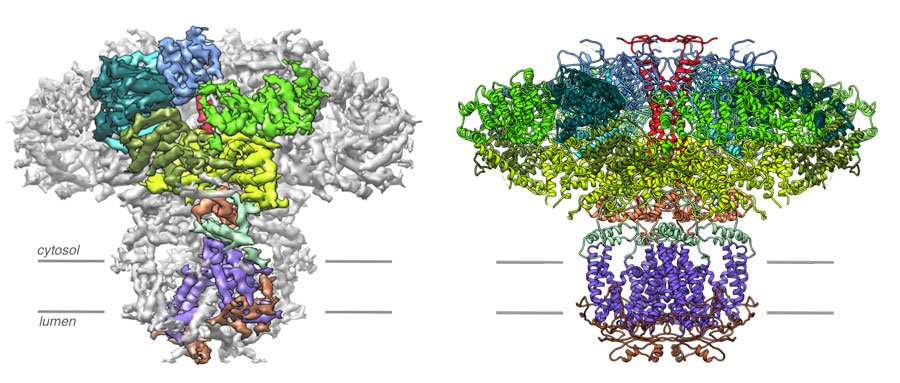
Left, a 4.7 Å resolution cryo-EM density map of the IP3R . Densities corresponding to the individual domains of one subunit are color coded. Right, the resulting atomic model constructed from the 4.7 Å resolution map of IP3R color coded by domain. Views are along the membrane plane.
