Injection Series: Part I – Hyaluronic Acid (HA)
Photo Credit: Freepik
A primary focus for Medicare auditors in 2024-2025 will be on pain management injections. In our injection series, we will delve into four types of pain management injection therapies. These include therapies on the Novitas TPE MAC radar, the active OIG Work Plan, or both. We will begin our series with HA (hyaluronic acid) injections, which are highlighted in both medical reviews. Interestingly, viscosupplementation has been classified as a device by the FDA, and Medicare has recognized it as a non-pharmacologic therapy.
Hyaluronic Acid Injections
Hyaluronic acid (HA) injections, also known as viscosupplementation, are a treatment option for knee pain caused by (OA) osteoarthritis. During the procedure, a healthcare provider injects a gel-like HA into the knee joint to help reduce pain and swelling. HA is a natural substance that acts as a lubricant and shock absorber in the joints, allowing bones to move smoothly against each other. People with OA have lower-than-normal levels of HA in their joints, which can contribute to pain when moving the knee.
Medicare’s top two TPE denials for HA injections are as follows:
Finally, HA injections are contingent on a diagnosis of pain associated with Osteoarthritis (OA) and are subject to limitations on how frequently they can be administered (see below).
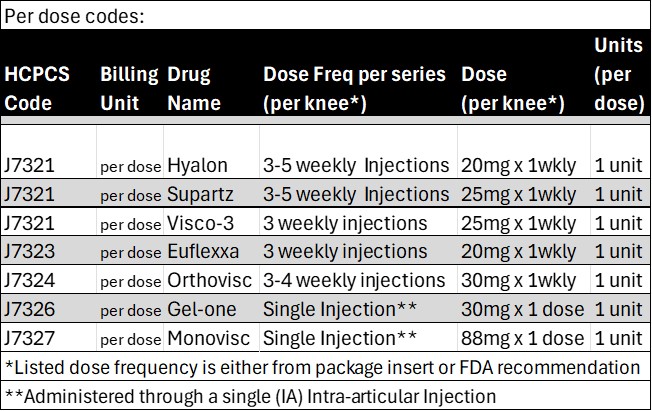
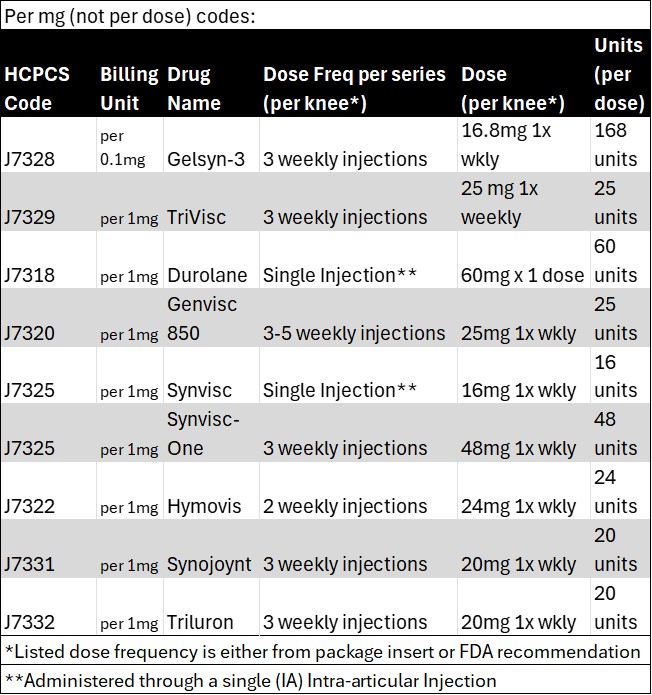
Per CMS articles, several modifiers are required for billing purposes. I have cited LCD A56157 information below, as well as provided (internal use only) links for the JW/JZ modifiers.
Billing subsequent injections in a series (EJ modifier)
A series is defined as a set of injections for each joint and each treatment. The EJ modifier must be used with the HCPCS code for the drug administered to indicate subsequent injections of a series. The modifier is not to be used with the first injection of each series.
Drug Waste Documentation for Single Dose/Use (PPT) (Documentation Tip Sheet)
Next in our series, Injection Series: Part II – Facet Joints (Paravertebral)
Resources
Novitas TPE (Target, Probe, Educate): HA Injections Checklist
LCD L39529: Intra-articular Knee Injections of Hyaluronan
LCD Article A56157 (updated 08/01/2024)