Venous Thromboembolism Prophylaxis
Original Date: 4/2003 | Supersedes: 01/2019, 10/2022 | Last Review Date: 3/2024
Purpose: To define patient populations at increased risk for VTE and administer appropriate prophylaxis.
Recommendations for VTE Prophylaxis:
- For trauma patients, provide low-molecular-weight heparin (LMWH) (Grade 2C) or unfractionated heparin (UH) (Grade 2C) and mechanical prophylaxis, preferably with intermittent pneumatic compression, unless contraindicated.
- For major trauma patients in whom LMWH and UH are contraindicated, mechanical prophylaxis, preferably with IPC, over no prophylaxis (Grade 2C) when not contraindicated by lower-extremity injury. Adding pharmacologic prophylaxis with either LMWH or UH when the risk of bleeding diminishes or the contraindication to heparin resolves (Grade 2C)
VTE Prophylaxis Drug Dosing:
| enoxaparin | ||
|---|---|---|
| Patient Weight | Renal Function | Drug Dosing |
| < 45 kg | CrCl> 30 ml/min | enoxaparin 20mg subcutaneous q12h |
| 45 kg – 89 kg | enoxaparin 30mg subcutaneous q12h | |
| 90 kg – 129 kg | enoxaparin 40mg subcutaneous q12h | |
| ≥ 130 kg | enoxaparin 50mg subcutaneous q12h | |
| unfractionated heparin (UH) | ||
|---|---|---|
| Patient Weight | Renal Function | Drug Dosing |
| < 90 kg | CrCl < 30 ml/min | heparin 5000 units subcutaneous q8h |
| ≥ 90 kg | heparin 7500 units subcutaneous q8h | |
Anti-factor Xa Dosing in ICU and IMU Patients:
For STICU patients, check an anti-factor Xa level 4 hours after the third dose of enoxaparin (1300 or 0100). The goal anti-factor Xa level for prophylaxis is 0.2-0.4. If the anti-factor Xa level is low, increase enoxaparin dose by 10 mg and recheck after the third new dose.
Timing of Chemoprophylaxis:
Dosing schedule (Entire Trauma Service Line)
- Enoxaparin: Administer at 0900 and 2100 schedule
- Heparin: Administer at 0600, 1400, and 2200 schedule
- Select “q8h-06” frequency to minimize night-time awakenings
To avoid missed doses, initiate VTE prophylaxis at the earliest time based on the above schedule for all trauma patients.
- Example: Patient arrives in trauma bay at 0600, start enoxaparin at 0900 after excluding TBI and spine injury requiring emergent operation
Contraindications to start immediate chemoprophylaxis:
Solid Organ Injury:
- Non-operative management of spleen, liver, and kidney injuries (ALL grades)
- Start VTE prophylaxis within 12 hours from hospital arrival, using the above administration schedule (at the first 0900/2100 following exclusion of TBI and spine injury requiring emergent operation)
- Patient may still be in ED at time of prophylaxis administration. Please order medication in MAR and ask nurse to administer in a timely fashion.
- If a patient undergoes angiography or operative repair of a solid organ injury, that injury is considered resolved and VTE chemoprophylaxis can begin immediately if:
- The angiography shows no extravasation/pseudoaneurysm
- The extravasation/pseudoaneurysm is successfully embolized
- The bleeding is controlled surgically
- VTE chemoprophylaxis should be held for patients with bleeding controlled with surgical packing until transfusion requirements decrease and coagulopathy resolves (within 24 hours ideally)
Traumatic brain injury:
- VTE prophylaxis to begin 24 hours after stable CT head
- VTE prophylaxis to begin 24 hours after craniotomy
- Chemoprophylaxis does not need to be held for EVD or ICP placement or removal.
Spine fractures and spinal cord injuries:
- Final spine disposition should occur within 6 hours from consult
- Will include operative vs non-operative plan and time of operation
- Non-operative spine trauma patients
- Begin chemical DVT prophylaxis per protocol
- If epidural/subdural hematoma present on MRI, begin chemical VTE prophylaxis per protocol after 24 hours from time of injury
- Operative spine trauma patients
- Hold AM dose of chemical VTE prophylaxis on day of surgery
- Patient may receive 9 pm enoxaparin dose the night before 0730 surgery start but hold doses on day of surgery
- Post-operative resumption of chemical VTE prophylaxis:
- With decompression/laminectomy/intradural procedures
- Hold 24 hours after procedure stop time
- Enoxaparin: please state “spine surgery” and specify start time of 0900 or 2100 (first available time after 24 hours) in comment section of order.
- Heparin: please state “spine surgery” and specify start time of 0600, 1400, or 2200 (first available time after 24 hours) in comment section of order.
- Order q2 hour neuro checks for the first 24 hours after initiation of chemical DVT prophylaxis
- Stabilization with instrumentation/fusion only
- At next scheduled dose – do not hold post-operatively
- With decompression/laminectomy/intradural procedures
- Patients will remain on Trauma or Neurosurgery service in the pre-operative period and 48 hours post-operatively
- Hold AM dose of chemical VTE prophylaxis on day of surgery
- After discharge, spinal cord injury patients or spine fracture patients that are immobile should continue chemical DVT prophylaxis with daily 325 mg aspirin for 12 weeks postoperatively.
- Exceptions to this protocol will exist and should be communicated between faculty members.
Chemical VTE prophylaxis in trauma patients is NOT routinely held in the following circumstances:
- Interventional radiology (IR) procedures (except solid organ biopsy)
- Orthopedic OR procedures (excluding spine)
- OR procedures – please do not wait 12 hours after surgery to resume VTE prophylaxis
- Fascia iliaca regional blocks with hip/femur fractures
Lab abnormalities and holding chemical VTE prophylaxis:
- Decision to hold medication on trauma service patients should be discussed with the chief resident, fellow, or attending
- Hemoglobin – no cutoff value for Hb, please continue medication in absence of active bleeding
- Platelets < 50,000 – consider holding chemoprophylaxis if acute thrombocytopenia with concern for HIT or active bleeding
- Platelets < 100,000 will prompt phone call from nurse – this does not mandate holding in absence of above criteria
Regional anesthetic with epidural catheter:
- If a pain consult is obtained for epidural placement, hold enoxaparin for 12hrs prior to epidural catheter placement and removal.
- While the patient has an epidural in place, the preferred dose of enoxaparin is 40mg subcutaneous q24h.
Background:
As a population at high risk for venous thromboembolism (VTE), early, aggressive thromboprophylaxis is warranted for trauma patients – with the risk of VTE rising sharply if treatment is delayed beyond 72 to 96 hours. Even with appropriate prophylaxis, VTE remains a leading cause of mortality in the trauma population. The optimal approach to thromboprophylaxis in patients with specific traumatic injuries remains incompletely defined
High risk patients are those anticipated to be hospitalized > 24 hours and have one or more of the following risk factors:
- Anticipated immobilization > 2 days
- Previous history of DVT, PE or hypercoagulable disease
- Head injury with GCS < 8 or unable to respond to commands
- Pelvic fracture
- Long bone fracture
- Spinal fracture
- Lower extremity venous injuries
- Cancer
- Obesity
- Multiple rib fractures
Mechanical DVT Prophylaxis
Thrombo-Embolic Deterrent (TED) hose and Sequential Compression Devices (SCDs) should be used for all high-risk patients.
Contraindications to initial TED hose and SCDs include:
- Non-fixated fractures
- Large open extremity wounds
- Extremities with external fixators or splints
SCDs may be used on fractured extremities following open reduction and internal fixation.
Arterial venous foot pumps (Plexi-Pulse) can be used if the foot is not injured.
Chemical DVT Prophylaxis
Chemical VTE prophylaxis should be used for all high-risk patients, with LMWH preferred over UH.
Relative contraindications to chemical VTE prophylaxis include:
- On-going blood loss
- Coagulopathy
- Thrombocytopenia
- History of heparin induced thrombocytopenia
- Alternative chemoprophylaxis should be used (discussed above)
Institution specific background:
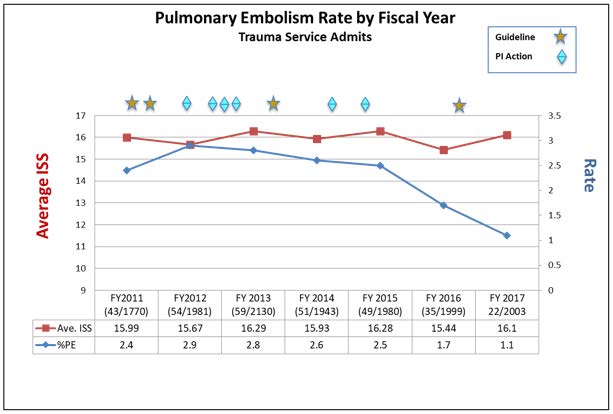
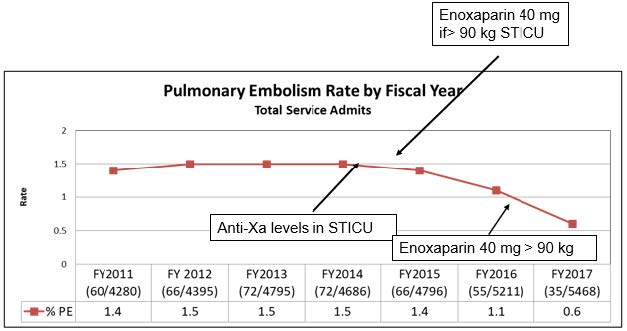
Given high rates of VTE on previous quality reports, multiple QI projects have been instituted to decrease the rate of VTE:
- Enoxaparin dose sent to OR with patient if they are to be in the operating when a dose is due (June 2013)
- VTE prophylaxis added to operating room time out (July 2013)
- Enoxaparin started 24 hours (from 48 hours) after stable CT head in traumatic injury patients (November 2013)
- Anti-factor Xa level monitoring done in STICU patients (February 2015)
- Starting enoxaparin dose increased to 40 mg q12 hours in STICU patients weighing over 90 kilograms (July 2016)
- Starting enoxaparin dose increased to 40 mg q12 hours in all patients weighing over 90 kilograms (July 2016)
These projects led to a decrease in the rate of missed doses and a decrease in the rate of VTE. So, while some of the VTE practices (e.g. anti-factor Xa dosing) may not have strong evidence to support their use, we are unsure which components of the multiple QI projects affected change and will continue them all until high quality data is available.
Appendix A: Relevant Literature Search
Below are the results of a limited search for studies comparing different methods for DVT prophylaxis in the general trauma population and subgroup populations involving nonoperative management of solid organ injuries, spinal cord injuries, and traumatic brain injuries including: randomized clinical trials, prospective observational studies, and prospective observational studies using a historical control group. The search was limited as there are several systematic reviews published within the past decade, which were used to ensure no relevant study was missed. For details of the search strategy, please see Appendix A.
Table 1: Chemoprophylaxis in General Trauma Patients
| Author / Year | Study Type | Patients, n | Inclusion Criteria | Exclusion Criteria | Drug Dosage / Timing | Outcomes |
|---|---|---|---|---|---|---|
| Geerts, 1996 | RCT | 265 | Adult patients admitted for trauma
ISS >9 |
Expected brain death or discharge within 7 days
TBI Uncontrolled bleeding Coagulopathy Renal insufficiency Pregnant |
LDH Group: Heparin 5000U q12hLMWH Group: Enoxaparin 30mg q12hFirst dose within 36 hours of admit |
Overall DVT rates: LDH 60 / 136 patients (44.1%) LMWH 40 / 129 patients (31%) – LMWH relative risk reduction for overall DVT by 30% (P=0.014)Proximal DVT rates: LDH 20 / 136 patients (14.7%) LMWH 8 / 129 patients (6.2%) – LMWH relative risk reduction for proximal DVT by 58% (P=0.012)Major bleeding complications: LDH 1 / 136 patients (0.6%) LMWH 5 / 129 patients (2.9%) -no significant difference between groups (P=0.12) |
| Note: LMWH more effective than LDH in preventing VTE after major trauma. Preoperative chemoprophylaxis can be given safely without increased bleeding complications in either group. Limitations: LDH dosed q12h. | ||||||
| Bickford Kay, 2018 | RCT | 234 | Adult patients admitted for trauma at high risk for DVT | Solid organ injury ≥Gr III
TBI Acute or chronic renal insufficiency (GFR <30) On therapeutic anticoagulation or aspirin Expected brain death or discharge within 72 hours |
Standard therapy (ST) Enoxaparin 30mg q12hWeight based (WB) Enoxaparin 0.5 mg/kg q12hTime to first dose protocol not given |
Overall VTE Rates: WB 4 / 110 (3.6%) -all DVTST 12 / 124 (9.7%) -11 DVT, 1 PE *no significant difference between groups (P = 0.075)Average LMWH Dose: ST 30mg compared to WB 40mg (P=0.001) with average BMI 27 for both groups (P=0.7) |
| Note: Weight-based enoxaparin dosing for VTE prophylaxis in trauma patients may provide better protection than standard dosing. Limitations: Trend towards significance in overweight patients with study limited by median BMI 27 rather than obese patients. | ||||||
| Ginzburg, 2003 | RCT | 442 | Adult patients admitted for trauma at high risk for DVT
At least one leg and one arm available for an IPC device |
ICH
On therapeutic anticoagulation Coagulopathy Uncontrolled bleeding Expected brain death or discharge within 7 days Renal failure (Cr >3.4) Pregnant Unable to obtain BLE duplex Morbidly obese |
LMWH Group: Enoxaparin 30mg q12hFirst dose within 24 hours of admit Exceptions: -Withheld 12 hours before any surgery IPC Group: -Sleeve disuse was tolerated for up to 8 hours consecutively -longer intervals resulted in exclusion from the analysis |
Overall VTE Rates: LMWH: DVT 1 / 218 (0.5%) PE 1 / 218 (0.5%) IPC: DVT 6 / 224 (2.7%), PE 1 / 224 (0.4%) Subgroup ISS >19 VTE Rates: LMWH 0 / 74 (0%), PE 0 / 74 (0%) IPC 2 / 74 (2.7%), PE 0 / 74 (0%) *No significant difference in overall VTE, DVT or PE rates. No extracranial bleeding complication No deaths attributed to bleeding complications from enoxaparin |
| Note: IPC effective for VTE prophylaxis in trauma patients. Limitations: No data on % enoxaparin doses missed for surgery and time to initiation. | ||||||
| Stannard, 2006 | RCT | 200 | Adult blunt trauma with at least one: -AIS >3 and one or more long bone fx -Age >55 years and one or more long bone fx |
Expected brain death or discharge within 72 hours
Renal insufficiency Severe TBI, SCI, or ocular trauma On therapeutic anticoagulation Hx VTE Any contraindication to MRV Pregnant Uncontrolled bleeding |
Group A: Enoxaparin 30mg q12h -First dose within 48 hours of admit Group B: PlexiPulse foot pump on admit Enoxaparin 30mg q12h First dose on HOD#5 |
Overall VTE Rates: Group A: DVT 13 / 97 (13.4%), PE 2 / 97 (2%) -11 / 13 (85%) with large (>2cm) or occlusive DVT (11.3% overall) -Mean time to LMWH (34.2 hours in DVT+ compared to 32.3 hours in DVT- ; not significant)Group B: DVT 9 / 103 (8.7%), PE 0 / 103 (0%) -3 / 9 (33%) with large (>2cm) or occlusive clots (2.9% overall)*No significant difference in overall VTE, DVT or PE rates between groups except subgroup analysis large or occlusive DVTs occurred significant less in Group B (P=0.025) |
| Note: PlexiPulse foot pumps effective for VTE prophylaxis in trauma patients. Trend towards significance when mechanical and chemoprophylaxis modalities combined. Limitations: No data on % enoxaparin doses missed for surgery and time to initiation. | ||||||
| Knudson, 1996 | RCT | 202 | Adult blunt trauma with at least one: -ISS>10 or AIS >3 any category -Major pelvic fx -Lower extremity fx above the ankle -DVT hx -Acute venous injury -Age >50 years |
Pregnant
Prisoners Severe TBI or SCI Liver or spleen injuries NOM Coagulopathy Uncontrolled bleeding after 24 hours Hospital LOS < 5 days |
LMWH Group: Enoxaparin 30mg q12hFirst dose within 24 hours of admit Compression Group: Sequential gradient pneumatic compression (SCD) first choice Arteriovenous impulse (AVI) device applied if unable to wear SCD secondary to ex-fix, casting, or soft tissue injury |
Overall VTE Rates: LMWH: DVT 1 / 120 (0.8%), PE 0% SCD: DVT 1 / 61 (1.6%), PE 0% AVI: DVT 1 / 21 (4.8%), PE 0% Historical control: DVT 9.1% *No difference between LMWH and compression groups (P=0.567) but overall significant reduction in DVT compared to historical control with no prophylaxis (P=0.037)Complications: LMWH: 6 / 120 (5%) major bleeding complications -1 / 6 (17%) required reoperation for pelvic bleeding SCD: 2% had mild skin changes AVI: 15% had major skin changes / blistering |
| Note: Trend significance that LMWH more effective than SCDs and that both methods are more effective than foot pumps. Foot pumps better than no prophylaxis but have risk of major skin changes. Limitations: no subgroup analysis comparing LMWH vs. SCD vs. AVI (compared LMWH vs. compression only). Excluded TBI, SCI, and SOI NOM injuries. | ||||||
| Olson, 2015 | RCT | 436 | Adult patients admitted for trauma at high risk for DVT (ISS >9) | Expected brain death or discharge within 7 days
Pregnant Prisoners INR >1.2 BMI >40 Renal insufficiency (Cr >1.3) Transfer time to facility >24 hours |
UFH Group: Heparin 5000U q8hLMWH group: Enoxaparin 30mg q12hFirst dose within 72 hours of admit Exceptions: Ppx held morning of surgery and restarted within 24 hours postop |
Overall VTE Rates: UFH: DVT 8.2%, PE 0.5% LMWH: DVT 5.1%, PE 0%Risk difference 3.1% (-1.6% to 7.7%), Relative risk 1.61 (P=0.196) *Analysis of all patients, UFH was non-inferior (<10% difference) compared with enoxaparin Subgroup US screened per protocol: UFH: DVT 17.1%, PE 1% LMWH: DVT 10.7%, PE 0% Risk difference 6.5% (-2.9-15.8%), Relative risk 1.61 (P=0.179) *Analysis of US screened patients, the non-inferiority (<10% difference) of UFH was inconclusive No difference in adverse events Pharmaceutical cost for UFH ($2,809) ~20-fold lower than enoxaparin ($54,138) |
| Note: UFH TID dosing may be equivalent to LMWH BID dosing but of those screened for VTE per study protocol (only 50% study patients) there was up to 15.8% risk difference using UFH dosing. Limitations: High VTE rates among study population likely from screening protocol, late initiation (median 3d both groups) and holding prophylaxis for surgery (median % administered doses UFH 100% vs. LMWH 97.1%, P=0.445) – may have shown larger reduction in VTE rates and difference among groups. | ||||||
| Fuchs, 2005 | RCT | 227 | Adult trauma patients admitted with one of the following bony or ligamentous trauma: Spine Pelvis including acetabulum Femur, tibia, or ankle |
Polytrauma
Decompensated CHF Severe PAD CVA Liver failure Pregnant Known malignancy Paraplegia Hx VTE |
Arthroflow Group Heparin 5000U TID + Arthroflow device Control Group Heparin 5000U TID First dose evening prior to surgery or immediately postoperatively if emergent operation |
Overall VTE Rates: Arthroflow Group -DVT 4 / 111 (3.6%), PE 0% Control Group -DVT 29 / 116 (25%), PE 0% *Significant 9-times reduction in DVT rates (P<0.001) when combining UFH with mechanical prophylaxis compared to UFH alone *Arthroflow group showed less proximal and extended thrombi (2.7% vs. 20.7%, P<0.001) |
| Note: Significant reduction in overall DVT and proximal DVT rates when mechanical and chemoprophylaxis modalities combined. Limitations: limited study population (excluded polytrauma, ISS data not given for study population). | ||||||
| Cohn, 1999 | RCT | 66 | Adult patients admitted for trauma with one of following: Age >45 >2 days immobility Hx VTE Spine fx GCS <8 |
TBI
Uncontrolled bleeding On therapeutic anticoagulation Hx hypercoagulability Heparin allergy Malignant hypertension Liver or renal failure |
Heparin 5000U q12h
Enoxaparin 30mg q12h -First dose within 24 hours of admit |
Overall VTE Rates: LMWH: DVT 0 / 34 (0%), PE 0% UFH: DVT 2 / 32 (6.2%), PE 0% *No significant difference in DVT (P=0.493) or PE rates* No significant difference in bleeding complications |
| Note: UFH may be equivalent to LMWH in moderately injured patients (mean ISS 12). Limitations: UFH BID dosing and small sample size. | ||||||
| Norwood, 2001 | Prospective observational | 118 | Blunt traumatic mechanism
Hospitalization >72 hours ISS >9 with at least one high risk criteria for VTE Enoxaparin prophylaxis initiated <24 hours of admit with no interruption throughout hospitalization |
Spinal cord injuries
Coagulopathy Failure to obtain BLE duplex within 24 hours discharge Trauma / NSGY discretion high risk bleeding or ongoing bleeding |
Enoxaparin 30mg q12h
First dose within 24 hours of admit Exceptions: |
Overall DVT rates: -DVT 2 / 118 patients (2%) -PE 0 / 118 patients (0%)Traumatic brain injuries: -45 / 118 patients (47%) -8 / 45 patients (15%) required craniotomy for evacuation of intracranial hematomas within the first 24 hours after admission and before initiation of enoxaparin therapy Spinal fractures without SCI: -27 / 118 patients (23%) -7 / 27 patients (26%) had decompressive and/or stabilization procedures NOM Liver / Spleen Injuries: -22 / 118 patients (20%) treated nonoperatively -2 / 22 patients (9%) failed NOM (one grade III and one grade IV splenic injuries) No major bleeding complications attributed to enoxaparin therapy in the study group patients |
| Note: LMWH can be safely started <24hrs after admission in severely injured trauma patients with spine fractures and low grade spleen / liver injuries. LMWH can be used safely in TBI injuries >24hrs after admission. Limitations: No data on actual time to initiation LMWH in TBI patients. Small sample size among subgroup populations examined but no major bleeding complications attributed to LMWH among overall cohort. | ||||||
| Cothren, 2007 | Prospective observational | 743 | Adult patients admitted for trauma at high risk for DVT | On therapeutic anticoagulation | Dalteparin 5000U qd
-First dose once hemodynamically stable Exception: |
Overall DVT rates: -DVT 26 / 743 patients (3.9%) -PE 6 / 743 patients (0.8%)No extracranial bleeding complication No radiographic progression of TBI following LMWH No deaths attributed to bleeding complications from enoxaparin |
| Note: Dalteparin once-daily dosing has similar safety and efficacy compared to previous studies utilizing twice-daily enoxaparin. Once-daily dosing increased compliance at single institution (33% to 74%). LMWH can be safely started within 24hrs of admission for severely injury patients, continued regardless of need for invasive procedures and after stable CTH in TBI patients. Limitations: LMWH initiated mean 3.3 days after admission and only 74% compliance with daily regimen. No data breakdown of VTE or noncompliance among subgroup of patients including TBI 174 patients (23%) and SCI 143 patients (19%). | ||||||
| Singer, 2016 | Prospective observational compared to historical control | 127 | Adult patients admitted to ICU for trauma at high risk for DVT | ICU LOS <72 hours
Pregnancy Renal insufficiency (GFR <30) On therapeutic anticoagulation TBI |
Treatment Group Enoxaparin 30 mg q12hFirst dose within 72 hours of admit Anti-Xa Levels: Peak drawn 4 hours after third dose -“Low” <0.2 IU/mL -“Normal” 0.2-0.4 IU/mL -Increased 10mg and Anti-Xa rechecked (dosed by peak levels)Historical Control Heparin 5000U q8h |
Initial Anti-Xa Levels “Normal” 44 / 127 patients (34.6%) “Low” 83 / 127 patients (65%) *43% of initial “low” patients achieved prophylactic levels with mean dose 44mg *”Low” mean weight 89.3 +/- 22.6kg compared to “Normal” mean weight 82.5 +/- 13kgOverall VTE Rates: -10 / 127 patients (7.9%) -SPL in 9 / 10 VTE patients (90%)High risk patients (RAP score ≥10) Treatment Group VTE 9 / 56 (16.1%), DVT 4 / 56 (7.1%), PE 5 / 56 (8.9%) Historical control -VTE 20 / 83 (24.1%), DVT 17 / 83 (20.5%), PE 3 / 83 (3.6%) *Significant decrease in DVT 20.5% to 7.1% (P=0.031) but not in overall rate of VTE or PE |
| Note: Low Anti-Xa levels are associated with higher DVTs rates and obese patients (“Low” mean weight 89.3+/-22.6kg and BMI 29.6 compared to “Normal” mean weight 82.5+/-13kg and BMI 27.5). Obese patients may benefit starting at LMWH 40mg BID initially and monitoring Anti-Xa levels for appropriate levels. Limitations: historical control used UFH TID dosing – unknown if dosing LMWH by anti-Xa or LMWH alone made VTE difference. | ||||||
| Louis, 2014 | Prospective observational | 202 | Adult patients admitted for trauma | Any known thromboembolic diagnoses
Any use of anticoagulants other than enoxaparin |
Enoxaparin dosing and timing not included in protocol, divided groups into:
Uninterrupted Group: Interrupted Group: *Median time to first dose = 2 days |
Overall VTE Rates: Uninterrupted: DVT 4.8% Interrupted: DVT 23.5% *Significantly more likely to develop DVT with interrupted prophylaxis (P<.01) *Patients not diagnosed with DVT were much less likely to miss doses than those diagnosed with DVT (50.8% vs 88.2%) (P>.001)Direct correlation between number of missed doses and risk of DVT starting at 2 doses missed: 1 ; OR 0.75 5-8 ; OR 10 2-4 ; OR 8.5 9-17 ; OR 14.7 |
| Note: DVT chemoprophylaxis should not be held for surgery or interrupted unnecessarily – increasing VTE rates directly correlate with increasing missed LMWH doses. Limitations: 50% patients in trial included general surgery patients but no difference in demographics or data otherwise. | ||||||
| Malinoski, 2010 | Prospective observational | 54 | All trauma patients admitted to SICU | Renal insufficiency
High bleeding risk Early mobilization Thrombocytopenia (<100k) |
Enoxaparin 30mg q12h
-First dose as soon as acute blood loss stabilized at discretion of attending surgeon Anti-Xa Levels: |
Anti-Xa Trough Levels / VTE Rates Normal levels 27 / 54 (50%) -DVT 3 / 27 (11%), PE 0 / 27 (0%) Low levels 27 / 54 (50%) -DVT 10 / 27 (37%), PE 1 / 27 (4%) *Low levels significantly more DVTs than pts with normal levels (37% vs. 11%, P=0.026) *Low trough levels significantly lower peak levels than pts with normal levels (0.17 IU/mL vs. 0.27 IU/mL, P<0.001) but peak levels were not significant different among patients with and without DVTs |
| Note: Standard dosing of enoxaparin leads to low anti-Xa trough levels in 50% SICU patients and is associated with a significant increase in the risk of DVT compared to those with normal levels (37% vs. 11%, P=0.026). Adjusting LMWH dosing by trough levels may correlate with VTE reduction more than peak levels – low trough levels correlated with an increase in DVTs compared to peak anti-Xa levels were not different in those with or without DVTs (0.23 IU/ml for both). Limitations: no control group to compare VTE rates. | ||||||
| Costantini, 2013 | Prospective observational | 61 | Adult patients admitted for trauma | Pregnant
Renal insufficiency (GFR <30) On therapeutic anticoagulation Missed screening duplex or anti-Xa levels |
Enoxaparin 30mg q12h
-Time to first dose protocol not given Anti-Xa Levels: |
Anti-Xa Peak Levels / VTE Rates Normal levels 18 / 61 (29.5%) -DVT 2 / 18 (11.1%), PE 0 / 18 (0%) Low levels 43 / 61 (70.5%) -DVT 0 / 43 (0%), PE 1 / 43 (2.3%) *No significant difference in DVT (P=0.0836) or PE (1.0) between groups *Low levels more likely male (P=0.0013), higher weight (70.5kg vs 87.5kg mean, P=0.0117), and higher BMI trended towards significant (BMI 25 vs. 28.7 mean, P=0.0558)“Low” dose changes 27 / 43 (63%) -22 / 27 (81.5%) achieved “normal” with 40mg q12h dosing *No patient who was initially “low” and received dose adjustments developed VTE *Trough plasma anti-Xa levels did not correlate well with peak anti-Xa levels |
| Note: Standard dosing of enoxaparin leads to low anti-Xa peak levels in 70% trauma patients but was not associated with significant difference in VTE rates compared to normal peak levels. Obese patients were more likely to have low levels and may benefit starting at LMWH 40mg BID initially (80% were corrected) and monitoring Anti-Xa levels for appropriate levels. Limitations: no control group to compare VTE rates. | ||||||
| Arnold, 2010 | Prospective observational | 476 | Adult patients admitted for trauma >72 hours | None listed | LMWH Group: -Enoxaparin 40mg qd OR 30mg q12hHeparin 5000U q8h-Time to first dose protocol not given Primary prophylaxis changed from LMWH to UFH mid-year |
Overall VTE rates: LMWH -DVT 16 / 237 (6.75%), PE 1 / 237 (0.42%) UFH -DVT 17 / 239 (7.11%), PE 3 / 239 (1.26%) *No difference in DVT (P=0.663) or PE (P=0.623) rates between groups Major Bleeding Complications LMWH 9 / 237 (3.8%) UFH 3 / 239 *1.2%) *No difference between groups (P=0.09) |
| Note: UFH TID dosing may be equivalent to LMWH. Limitations: used LMWH once daily (unknown % patients) and 61% of DVTs diagnosed in TBI / SCI patients with mean time to initiated prophylaxis >6 days in both groups. No data examining subgroups. | ||||||
| Ko, 2016 | Prospective observational compared to historical control | 205 | Adult patients admitted for trauma | LOS < 2 days
Preexisting DVT |
Adjustment Group: Enoxaparin 30mg q12hAnti-Xa Levels: Trough drawn 30 minutes to 1 hour before the fourth dose -“low” ≤0.1 IU/mL -Increased 10mg and Anti-Xa rechecked (dosed by trough levels)Historical Control: Enoxaparin 30mg q12h without anti-Xa adjustment-Time to first dose protocol not given |
Anti-Xa Trough Levels / VTE Rates Adjustment Group -“low” 73 / 87 (83.9%) -78% achieved “normal” levels with 40mg q12h -VTE 1.1%: DVT 1 / 87 (1%), PE 0 / 87 (0%) Control Group -VTE 7.6%: DVT 8 / 118 (6.8%), PE 1 / 118 (0.8%) *No significant difference in DVT (P=0.51) or PE (P=0.99) but overall VTE were lower (P=0.46) in Adjustment group despite more severely injured patient (ISS median 17 vs. 10, P=0.01), more spine fractures (28.7% vs. 16.9%, P=0.04), and ICU LOS (14.8d vs. 11.5d, P=0.009). Time to initiation LMWH did not differ (mean 2.6 days).*No significant difference in bleeding complications or PRBC transfusions |
| Note: Adjusting LMWH dosing by Anti-Xa trough levels reduces overall VTE rates compared to standard LMWH dosing. Limitations: used historical control and study population median BMI 25 (may have decreased overall risk difference) | ||||||
Table 2: Chemoprophylaxis in TBI Patients
| Author / Year | Study Type | Patients, n | Inclusion Criteria | Exclusion Criteria | Drug Dosage / Timing | Outcomes |
|---|---|---|---|---|---|---|
| Phelan, 2012 | RCT | 62 | Adult patients admitted with TBI | Moderate or High risk TBI
Grade IV / V solid organ injuries Spinal canal hematoma Uncontrolled bleeding Expected brain death or discharge within 48 hours Renal insufficiency Coagulopathy Pregnant |
Enoxaparin 30mg q12h
Placebo q12h -First dose started 24 hours after admit |
Radiographic TBI progression: Enoxaparin 2 / 36 patients (5.9%) Placebo 1 / 28 patients (3.6%) *Treatment effect difference of 2.3% (95%CI, -14.4%-16.5%, not statistically significant) *patients with progression stopped treatment, required no further intervention and all discharged home with GCS15 -CTH obtained 24hrs after initiation study drug (48hrs after injury)No clinical TBI progressions No extracranial bleeding complication *Mean time to initiation LMWH |
| Note: Starting LMWH 24hrs after admission in mild TBI patients appears safe. Mild TBI patients have natural progression of TBI bleed in ~3.6% placebo patients but all were subclinical. Limitations: Excluded moderate/severe TBI patients. | ||||||
| Kurtoglu, 2004 | RCT | 120 | Adult patients admitted with TBI or spine trauma requiring ICU | Liver or renal failure
SCI Hx VTE On therapeutic anticoagulation Coagulopathy Increase ICH repeat CTH 24 hours after admit or patients requiring craniotomy |
Intermittent pneumatic compression (IPC Group)
Enoxaparin 40mg qd -First dose timing determined by trauma/NGSY team following routine CTH 24hrs after admit |
Overall VTE / Mortality Rates: IPC Group: DVT 4 / 60 (6.6%), PE 2 / 60 (3.3%), Mortality 7 / 60 (11.6%) -2 / 7 deaths were from PE LMWH Group: DVT 3 / 60 (5%), PE 4 / 60 (6.6%), Mortality rate 8 / 60 (13.3%) -4 / 8 deaths were from PE *No significant difference in DVT (P=0.07), PE (P=0.07), and mortality (P=0.08)Radiographic TBI Progression: IPC 1 / 60 (1.7%) LMWH 1/ 60 (1.7%)Major Bleeding Complications: LMWH Group: mean 2.8 units IPC Group: mean 0.9 units *LMWH higher transfusion rate (P=0.03) -4 liver (3 Gr2, 1 Gr3), 3 spleen (2 Gr2, 1 Gr3) injuries managed NOM in LMWH group No patient required discontinuation of therapy and no deaths attributed to bleeding |
| Note: Starting LMWH 24hrs after admission in TBI / spine trauma patients appears safe. IPC is effective in overall VTE reduction compared to no prophylaxis in high risk patients. Radiographic TBI progression appears unchanged with LMWH but require slightly more blood transfusions. Limitations: Did not stratify TBI severity. No data on time to initiation lovenox and used once daily dosing lovenox. | ||||||
| Norwood, 2002 | Prospective observational | 150 | Adult patients admitted with TBI | Coagulopathy
Heparin allergy Expected brain death or discharge within 48 hours |
Enoxaparin 30mg q12h
-First dose 24 hours after admit Exceptions: -24 hours after craniotomy or cranioplasty *Did not withhold prophylaxis for ventriculostomies, liver injuries, retroperitoneal injuries or pelvic injuries. |
Radiographic TBI progression: Overall: 34 / 150 patients (23%) Pre-enoxaparin 28 / 34 patients (82%) -28 / 150 (19% overall) -no patients progressed after starting prophylaxis on follow up imaging Post-enoxaparin 6 / 34 patients (18%) -6 / 150 (4% overall) -prophylaxis discontinued without further progression on follow up imaging *statistically significant decrease in rate of ICH progression observed after 24 hours and after initiation of enoxaparin therapy (P=0.002)Postoperative Bleeding: Craniotomies 24 / 150 patients (16%) -2 / 24 (8%) major bleeding requiring reoperation *Both complications occurred early in study, protocol adjusted to start enoxaparin 24 hours postoperatively -Remaining 22 / 24 (92%) after protocol changed had no postoperative complications Major Bleeding Complications: Nonoperative TBI 126 / 150 (84%) -4 / 126 (3%) major bleeding complications (1 require operation) *All protocol violations (LMWH started 12hrs or double-dosed) Overall VTE Rates: No deaths attributed to bleeding complications from enoxaparin |
| Note: Starting LMWH 24hrs after admission in TBI patients appears safe. LMWH should be held 24hrs following craniotomy or cranioplasty. LMWH can be continued during ventriculostomy placement and removal without adverse effect. Radiographic TBI progression occurs naturally in up to 19% TBI patients and LMWH appears to be safe despite initial progression. Major bleeding occurred in 3% patients and rarely requiring operative intervention. Limitations: no control group. | ||||||
| Norwood, 2008 | Prospective observational | 525 | Adult patients admitted with TBI | Coagulopathy
Heparin allergy Expected brain death or discharge within 48 hours Solid organ injuries Spinal canal hematomas |
Enoxaparin 30mg q12h
-First dose within 24-48 hours of admit Exceptions: *LMWH held 12 hours preoperatively and 24 hours postoperatively for all cranial operations and 12 hours before ventriculostomy removal |
Radiographic TBI progression Overall: 62 / 525 patients (12%) Pre-enoxaparin -44 / 525 patients (8.3%) Post-enoxaparin -18 / 525 patients (3.4%) -10 / 18 patients (56%) were protocol violations (given early <24 hours) *If excluded protocol violations, post-enoxaparin = 8 / 443 patients (1.8%)Overall DVT Rates: DVT 6 / 525 patients (1.14%) PE 0 / 525 patients (0%)No deaths attributed to bleeding complications from enoxaparin No extracranial bleeding complication Time to initiation LMWH median time of 25.5 hours |
| Note: Starting LMWH 24hrs after admission in TBI patients appears safe. Limitations: Selection bias, despite meeting study criteria patients not enrolled due to surgeon bias (n=365, 24.4%) or unknown (n=289, 19.3%). No control group. | ||||||
| Nickele, 2013 | Prospective observational compared to historical control | 135 patients | Adult patients admitted with TBI | Pregnancy
Coagulopathy Uncontrolled bleeding Expected brain death or discharge within 48 hours *Faculty preference |
Heparin 5000U q12h -or- Dalteparin 5000U qd – First dose 24 hours following two consecutive CT scans at least 3 hours apart showing stability |
Historical Control (Faculty Preference) -22 / 48 patients (45.8%) received DVT prophylaxis -Average time first dose = 4.9 days -DVT 2 / 48 patients (4.2%) -PE 2 / 48 patients (4.2%)Intervention Group -63 / 87 (72.4%) received DVT prophylaxis -Average time first dose = 3.4 days -DVT 6 / 87 patients (6.9%) -PE 5 / 87 patients (5.7%) *No significant difference in DVT (P=0.2) or PE *% patients receiving prophylaxis significantly increased with institutional protocol (p<0.0001) (P=0.45) rates No deaths attributed to bleeding complications from enoxaparin No extracranial bleeding complications or radiographic progression of bleeds after LMWH |
| Note: Institutional protocols increase % compliance with VTE prophylaxis. Limitations: despite protocol, high non-compliance remained and delay in prophylaxis likely led to no significant difference. Used UFH BID dosing and dalteparin once daily dosing. | ||||||
| Pahatouridis, 2010 | Prospective observational | 61 | Adult patients admitted with Moderate TBI (GCS 9-12) | *Injury of another system
Coagulopathy On anticoagulant therapy |
Tinzaparin 3500 IU anti-xa qd
-First dose within 24 hours of admit |
No subclinical radiographic TBI progression or clinical TBI progression after LMWH
No deaths attributed to bleeding complications from enoxaparin No extracranial bleeding complication |
| Note: Starting LMWH 24hrs after admission in Moderate TBI patients appears safe. Limitations: Small sample size with no control group and unknown % compliance with protocol or time to initiation. | ||||||
| Samuel, 2015 | Prospective observational | 398 | Adult patients admitted with TBI or SCI weighing >100 kg | On anticoagulant therapy | Traditional dose group (TDG) Heparin 5000U q8hHigh dose group (HDG) Heparin 7500U q8h-Time to first dose protocol not given |
Overall VTE Rates: HDG 8 / 141 patients (5.7%) overall -DVT 6 / 141 patients (4.3%) -PE 2 / 141 patients (1.4%) -Mortality 13 / 141 patients (9%) TDG 24 / 257 patients (9.3%) overall -DVT 19 / 257 patients (7.4%) -PE 6 / 257 patients (2.3%) -Mortality16 / 257 patients (6%) *No significant difference in overall VTE (P=0.20), DVT (P=0.22), or PE (P=0.53), or mortality (P=0.55) rates between groups *Significant higher body weight in HDG (123kg) compared to TDG (116kg), P<0.01 but early time to first dose (HDG 34hrs compared to LDH 71hrs, P<0.01) and earlier postop (HDG 29hrs compared to LFH 55hrs, P<0.01).Time to heparin and VTE dx: Day 1, 3.9% (3 / 76) Day 2, 3.6% (4 / 112) Day 3, 5.8% (4 / 69) ≥Day 4, 16% (23 / 114) *No significant difference between days 1-3 compared to 2.75 fold increase after day 4 compared to day 3 (5.8% vs. 16%, P=0.02) No significant differences in adverse events, bleeding, or progression brain bleed on CT scans |
| Note: Delaying VTE prophylaxis beyond 3 days significantly increases VTE rates. Higher heparin doses appear safe and may benefit obese patients with an absolute risk reduction of 4% and relative risk reduction of 40% in VTE rates – although not statistically significant and HDG had earlier time to initiation than TDG. Limitations: no protocol on time to initiation heparin and unequal comparison groups (higher weights and earlier initiation in HDG). | ||||||
| Kim, 2002 | Prospective observational | 64 | Adult patients admitted with Severe TBI (AIS>3) | Coagulopathy
On therapeutic anticoagulation Died within 72 hours of admission Received LMWH for VTE ppx |
Heparin 5000U q12h
First dose: |
Overall VTE Rates: Early: DVT 2 / 47 (4%), PE 2 / 47 (4%), Death 4 / 47 (9%) Late: DVT 1 / 17 (6%), PE 0 / 17 (0%), Death 1 / 17 (6%) *No significant difference DVT (P=1.0), PE (P=0.96), or Death (P=1.0)*Early group subgroup analysis: -32 / 47 (68%) received UFH within 24 hours -5 / 47 (10.6%) had BCVI requiring IV heparin with PTT 50-80 (no systemic bleeding complications) -9 / 47 (19%) had liver / spleen injuries No deaths attributed to bleeding complications from enoxaparin No extracranial bleeding complication |
| Note: Starting VTE prophylaxis within 72 hours of admission appears safe in severely injured patients (Early group compared to Late group ISS 30 vs. 35, P=0.1303) with severe TBI. VTE prophylaxis was safely started within 24 hours of admission in 68% of Early group patients. Patients with concomitant BCVI injuries requiring systematic anticoagulation may be safely treated without systemic bleeding complications if monitored closely. Limitations: Selection bias, no randomization between groups although TBI severity, multi-system injuries or demographics did not differ between groups. UFH dosed BID. | ||||||
Table 3: Chemoprophylaxis in Solid Organ Injury (SOI) Patients
| Author / Year | Study Type | Patients, n | Inclusion Criteria | Exclusion Criteria | Drug Dosage / Timing | Outcomes |
|---|---|---|---|---|---|---|
| Schellenberg, 2019 | Prospective Observational | 118 | Adult patients admitted with non-operative blunt solid organ injury | Ed death
Transfers Home anticoagulation/antiplatelet therapy Did not receive VTE prophylaxis |
Enoxaparin (dose/frequency not described)
Heparin (dose/frequency not described) -First dose started <48 hours (early) after admit compared to >48 hours (late) |
Overall VTE Rates: Early: DVT 0 / 61 (0%), PE 2 / 61 (3%), Death 2 / 61 (3%) Late: DVT 5 / 57 (9%), PE 3 / 57 (5%), Death 1 / 57 (2%)*Significantly less DVT in early group (p=0.02) *No significant difference PE (P=0.67), or Death (P=1.0)No patients failed non-operative management No difference in volume of post-prophylaxis blood transfusion |
| Note: Starting VTE prophylaxis within 48 hours of admission appears safe (Early group compared to Late group had no difference in nonoperative failure or blood transfusions). VTE was less in the early prophylaxis group (DVT 0 vs 5, p=0.024; PE 2 vs 3, p=0.672). Limitations: only 118 patients in a single center study, no duplex surveillance, and no missed doses captured. | ||||||
Appendix B: Search Strategy
As there are a number of recent systematic reviews regarding this topic, a more limited search was performed, and the multiple systematic reviews were used to ensure that no relevant article was missed. Search limitations included: English language, randomized clinical trial, and prospective observational study (with or without historical control).
Chemoprophylaxis in General Trauma Patients
| Search | Database | Search Term | Limits | Total Yield: # of Articles | # Excluded Articles | # Included Articles |
|---|---|---|---|---|---|---|
| 1 | PubMed | trauma AND (heparin OR lovenox OR venous thromboembolism prophylaxis) |
Clinical Trial | 28 | 14 (12 unrelated) (2 risk factor/outcomes) |
14 (7 RCT) (7 Prospective observational) |
| 2 | “ | “ | Randomized Controlled Trial | 21 | 10 (10 unrelated) |
11 (7 RCTs) (4 Prospective observational) |
| 3 | “ | “ | Systematic Review | 20 | 9 (9 unrelated) |
11 |
| Total | 69 | 33 | 36 | |||
| Excluded: 31 unrelated, 2 risk factor / outcomes | ||||||
| Included: 14 RCTs (6 duplicates), 11 prospective observational (3 duplicates), 11 systematic reviews | ||||||
Chemoprophylaxis in TBI Patients
| Search | Database | Search Term | Limits | Total Yield: # of Articles | # Excluded Articles | # Included Articles |
|---|---|---|---|---|---|---|
| 1 | PubMed | traumatic brain injury AND (heparin OR lovenox OR venous thromboembolism prophylaxis) |
Clinical Trial | 8 | 4 (3 retrospective) 1 (unrelated) |
4 (4 Prospective observational) |
| 2 | “ | “ | Randomized Controlled Trial | 6 | 4 2 (secondary outcome) 2 (unrelated) |
2 (2 RCTs) |
| 3 | “ | “ | Systematic Review | 12 | 2 1 (duplicate) 1 (unrelated) |
10 |
| Total | 26 | 10 | 16 | |||
| Excluded: 3 retrospective, 4 unrelated, 2 secondary outcome, 1 duplicate | ||||||
| Included: 10 systematic reviews, 4 prospective observational, 2 RCTs | ||||||
Chemoprophylaxis in Solid Organ Injury Patients
| Search | Database | Search Term | Limits | Total Yield: # of Articles | # Excluded Articles | # Included Articles |
|---|---|---|---|---|---|---|
| 1 | PubMed | trauma AND (solid organ injury OR spleen OR liver) AND (heparin OR lovenox OR venous thromboembolism prophylaxis) |
Clinical Trial | 1 | 0 | 1 (1 Prospective observational) |
| 2 | “ | “ | Randomized Controlled Trial | 0 | 0 | 0 |
| 3 | “ | “ | Systematic Review | 1 | 0 | 1 |
| Total | ||||||
| Excluded: 0 | ||||||
| Included: 1 | ||||||
References
Geerts WH, Jay RM, Code KI, Chen E, Szalai JP, Saibil EA, Hamilton PA. A comparison of low-dose heparin with low-molecular-weight heparin as prophylaxis against venous thromboembolism after major trauma. N Engl J Med. 1996;335:701-707
Kay AB, Majercik S, Sorensen J, Woller S. Weight-based enoxaparin dosing and deep vein thrombosis in hospitalized trauma patients: A double-blind, randomized, pilot study. Surgery,Volume 164,Issue 1,144-149
Ginzburg E, Cohn SM, Lopez J, et al. Randomized clinical trial of intermittent pneumatic compression and low molecular weight heparin in trauma. Br J Surg 2003; 90:1338.
Stannard JP , Lopez-Ben RR , Volgas DA , et al . Prophylaxis against deep-vein thrombosis following trauma: a prospective, randomized comparison of mechanical and pharmacologic prophylaxis . J Bone Joint Surg Am . 2006 ; 88 ( 2 ): 261 – 266.
Knudson MM, Morabito D, Paiement GD, Shackleford S. Use of low molecular weight heparin in preventing thromboembolism in trauma patients. J Trauma. 1996;41:446-459.
Olson EJ, Bandle J, Calvo RY, et al. Heparin versus enoxaparin for prevention of venous thromboembolism after trauma: A randomized non-inferiority trial. J Trauma Acute Care Surg 2015; 79:961.
Fuchs S , Heyse T , Rudofsky G , Gosheger G , Chylarecki C . Continuous passive motion in the prevention of deep-vein thrombosis: a randomised comparison in trauma patients . J Bone Joint Surg Br . 2005 ; 87 ( 8 ): 1117 – 1122.
Cohn SM, Moller BA, Feinstein, AJ, Burns GA, Ginzburg E, Hammers LW. Prospective trial of low-molecular-weight heparin versus unfractionated heparin in moderately injured patients. Vascular Surgery 1999;33(2):219–23.
Norwood SH, McAuley CE, Berne JD, et al. A potentially expanded role for enoxaparin in preventing venous thromboembolism in high risk blunt trauma patients. J Am Coll Surg 2001; 192:161.
CC Cothren, WR Smith, EE Moore, et al. Utility of once-daily dose of low-molecular-weight heparin to prevent venous thromboembolism in multisystem trauma patients. World J Surg, 31 (2007), pp. 98-104
Singer GA, Riggi G, Karcutskie CA, Vaghaiwalla TM, Lieberman HM, Ginzburg E, et al. Anti-Xa guided enoxaparin thromboprophylaxis reduces the rate of deep venous thromboembolism in high-risk trauma patients. J Trauma Acute Care Surg. 2016;81:1101–1108.
Louis SG, Sato M, Geraci T, et al. Correlation of missed doses of enoxaparin with increased incidence of deep vein thrombosis in trauma and general surgery patients. JAMA Surg. 2014;149(4):365-370.
Malinoski D, Jafari F, Ewing T, Ardary C, Conniff H, Baje M, Kong A, Lekawa ME, Dolich MO, Cinat ME, et al. Standard prophylactic enoxaparin dosing leads to inadequate anti-Xa levels and increased deep venous thrombosis rates in critically ill trauma and surgical patients. J Trauma. 2010;68:874-880.
Constantini TW, Min E, Box K, Tran V, Winfield RD, Fortlage D, et al. Dose adjusting enoxaparin is necessary to achieve adequate venous thromboembolism prophylaxis in trauma patients. J Trauma Acute Care Surg. 2013;74:128–135.
Arnold JD, Dart BW, Barker DE, et al. Gold Medal Forum Winner. Unfractionated heparin three times a day versus enoxaparin in the prevention of deep vein thrombosis in trauma patients. Am Surg 2010; 76:563.
Ko A, Harada MY, Barmparas G, Chung K, Mason R, Yim DA, et al. Association between enoxaparin dosage adjustment by anti-factor Xa trough level and clinically evident venous thromboembolism after trauma. JAMA Surg. 2016;151:1006–1013.
Phelan HA, Wolf SE, Norwood SH, et al. A randomized, double-blinded, placebo-controlled pilot trial of anticoagulation in low-risk traumatic brain injury: The Delayed Versus Early Enoxaparin Prophylaxis I (DEEP I) study. J Trauma Acute Care Surg 2012; 73:1434.
Kurtoglu M, Yanar H, Bilsel Y, et al. Venous thromboembolism prophylaxis after head and spinal trauma: intermittent pneumatic compression devices versus low molecular weight heparin. World J Surg 2004; 28:807.
Norwood SH, Berne JD, Rowe SA, et al. Early venous thromboembolism prophylaxis with enoxaparin in patients with blunt traumatic brain injury. J Trauma 2008; 65:1021.
Nickele CM, Kamps TK, Medow JE. Safety of a DVT chemoprophylaxis protocol following traumatic brain injury: a single center quality improvement initiative. Neurocrit Care 2013; 18 (2) 184-192
D Pahatouridis, GA Alexiou, A Zigouris, E Mihos, D Drosos, S Voulgaris Coagulopathy in moderate head injury. The role of early administration of low molecular weight heparin. Brain Inj, 24 (2010), pp. 1189-1192
Samuel S, Iluonakhamhe EK, Adair E et al (2015) High dose subcutaneous unfractionated heparin for prevention of venous thromboembolism in overweight neurocritical care patients. J Thromb Thrombolysis 40:302–307.
Kim, J, Gearhart, MM, Zurick, A, et al. 2002Preliminary report on the safety of heparin for deep venous thrombosis prophylaxis after severe head injury J. Trauma 53:8-43.
Schellenberg M, Inaba K, Biswas S, Heindel P, Benjamin E, Strumwasser A, Matsushima K, Lam L, Demetriades D. When is It Safe to Start VTE Prophylaxis After Blunt Solid Organ Injury? A Prospective Study from a Level I Trauma Center. World J Surg. 2019 Nov;43(11):2797-2803. doi: 10.1007/s00268-019-05096-7. PMID: 31367780.
Murphy PB, de Moya M, Karam B, Menard L, Holder E, Inaba K, Schellenberg M. Optimal timing of venous thromboembolic chemoprophylaxis initiation following blunt solid organ injury: meta-analysis and systematic review. Eur J Trauma Emerg Surg. 2022 Jun;48(3):2039-2046. doi: 10.1007/s00068-021-01783-0. Epub 2021 Sep 18. PMID: 34537859.