Areas of Interest
Research Interests
Channelrhodopsins; membrane protein structure; ion channels; proteoliposomes
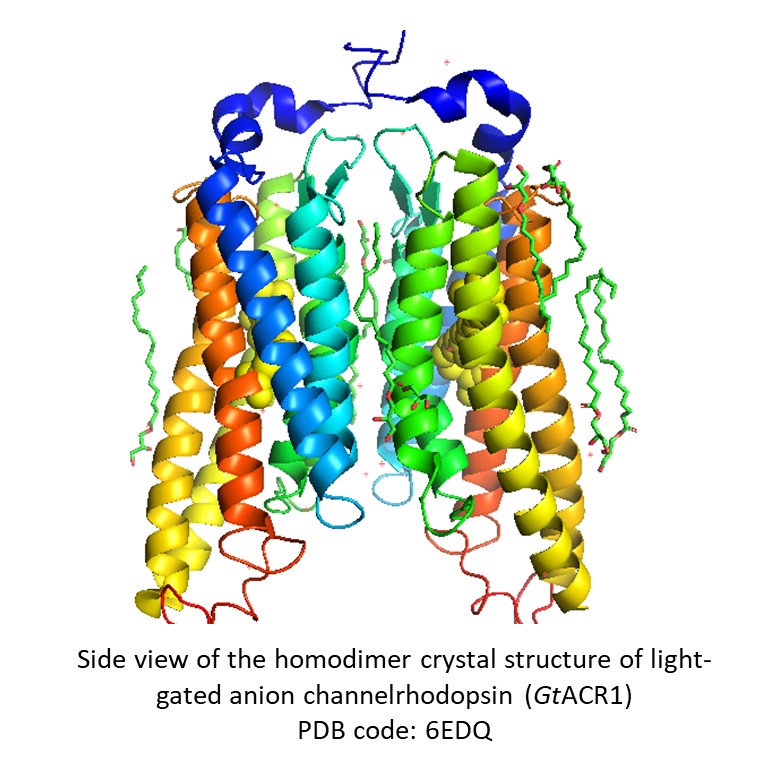
My current research is focused on understanding the mechanisms of channelrhodopsin (ChRs) function via structure determination and in vitro activity. ChRs are a subfamily of microbial rhodopsins that act as light-gated ion channels which conduct cations such as Na+ or anions such as Cl-. When heterologously expressed in animal cells such as neurons, they are used to control the membrane potential either by depolarization or hyperpolarization (optogenetics). However, the mechanisms underlying their function are not completely understood. I am mostly working on the structure determination with crystallography and Cryo-EM. Membrane proteins are more difficult to overexpress and purify than soluble proteins. I utilize different expression system including E. coli, Pichia pastoris, and insect cells to express various ChRs and purify with chromatography such as affinity, ion exchange, and size exclusion to obtain purified rhodopsin proteins in a large quantity. For crystallography, I set up crystallization trials with thousands of conditions using robot. In the collaboration with the lab in PSI Switzerland, we have determined the crystal structure of one anion ChR, GtACR1 to 2.9A (see left figure). From the structure we identified a complete continuous tunnel inside GtACR1 which we postulate to the Cl- conduction pathway. Furthermore by structure-directed mutagenesis, we confirm the role of residues lining along the pathway for Cl- conductance. For cryo-EM, I purified the proteins in high purity and homogeneity. In the collaboration with the lab in University of Toronto Canada, we determined the Cryo-EM structures of one K+-selective ChR, HcKCR1 and one Na+-selective ChR, HcCCR to ~2.6A (see right figure). Through the structures, we have identified the cavities which we postulate to the cation conduction pathway. By structure-directed mutation, we show that K+ versus Na+ selectivity is determined at two sites on the putative ion conduction pathway: in a patch of critical residues in the intracellular region and within a cluster of aromatic residues in the extracellular region.
My future goal is to continue to purify the ChRs and mutants and determine their crystal or cryo-EM structures and elucidate the molecular mechanism underlying their function.
Education and Training
BS
Biochemistry, Central China Normal University, Wuhan, China
PhD
Biochemistry, Old Dominion University, Norfolk, VA
Post-doctoral Fellow
Baylor College of Medicine
Post-Doctoral Fellow
McGovern Medical School, Department of Biochemistry and Molecular Biology
