About Integra Houston – HPTN 094
What is HPTN 094?
HPTN 094 or “INTEGRA” is a vanguard study to determine the effectiveness of using a mobile health unit to provide integrated health services – particularly medication for opioid use disorder (OUD) and medication for HIV treatment or prevention – to people with OUD who inject drugs in five U.S. cities.
Who is participating in HPTN 094?
People 18-60 years of age with opioid use disorder (OUD), who inject drugs, are at risk of acquiring HIV or are living with HIV and are willing to start OUD treatment are eligible to participate.
Why is Integra Houston important?
HPTN 094 will address the overlapping and intertwined epidemics of opioid addiction and HIV among people who inject drugs (PWID). Drug overdose is the leading cause of accidental death in the United States with more than 67,000 fatalities in 2018, but PWID face more than just the risk of overdose. The lack of access to health care (including medication for opioid use disorder), poverty, prevalent poly-substance use, and mental health disorders experienced by PWID combine to exacerbate the risk of HIV transmission and acquisition and other health issues. When they exist, the available health services needed to address the diverse health needs of PWID are often located far away from each other and from the PWID who need them, presenting serious barriers to access. Opioid use itself is a disorganizing factor in the lives of PWID, interfering with consistent access to healthcare, and the stigma or judgment PWID face from healthcare personnel cause many to avoid seeking care. HPTN 094 will meet people with OUD “where they are” by bringing integrated and judgment-free health services, supported by peer navigation, to PWID where they live.
What will happen during HPTN 094?
Eligible participants will be randomized to either the intervention arm or the active control arm.
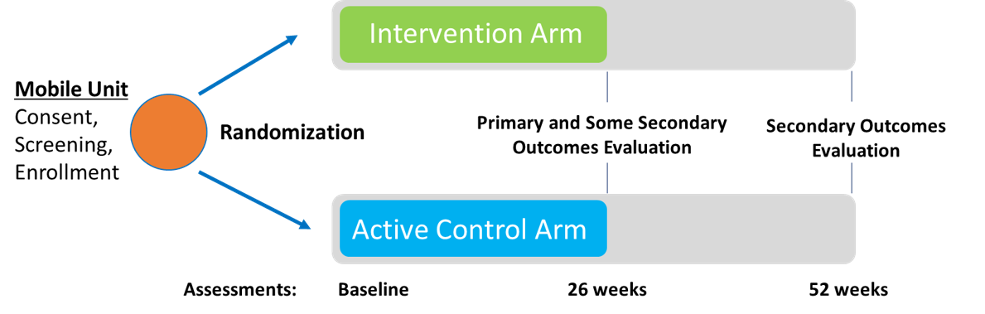
Intervention Arm: Participants will receive “one stop” health services in a mobile health unit and peer navigation from a peer recovery coach for 26 weeks. During this time, the mobile unit will provide the participant with primary care services including medication for opioid use disorder (MOUD), ART, PrEP and STIs as well as harm reduction services, and screening and referral for hepatitis, mental health issues, and other medical conditions. After 26 weeks, participants will be referred to health services available in the community.
Active Control Arm: Participants who are randomized to the active control arm will receive peer navigation to health services available in the community.