Bhanu P. Ganesh, PhD
- Associate Professor
- Director, Center for GUT-BRAIN Axis in Aging
- BRAINS Research Laboratory
- BIOME Group
Biography
Dr. Ganesh received her Ph.D. under the supervision of Dr. Michael Blaut from the German Institute of Human Nutrition-Potsdam Rehbruecke (DIfE), Leibniz institute, Germany from 2010 to 2014. Shortly thereafter, she relocated to Houston, TX for her post-doctoral training where she was trained by Dr. James Versalovic in the Department of Pathology and Immunology at Baylor College of Medicine from 2014 to 2017. She in-between held a visiting scientist position at Massachusetts Institute of Technology (MIT), Cambridge from 2014-2016 where she was trained by Dr. James Fox. After post-doctoral training at Baylor, she joined the department of Neurology (BRAINS lab) led by Dr. Louise McCullough at University of Texas Health Science Center Houston (UTHSC) as a senior postdoctoral fellow, Texas, USA from 2017 to 2018. In 2018, she was promoted to Assistant Professor in the department of Neurology, and in 2024, was promoted to Associate Professor with tenure. Currently, her primary interest lies on investigating signaling mechanisms involved in gut-brain axis interactions in aging-associated cerebrovascular diseases especially, stroke, Alzheimer’s disease, Cerebral Amyloid Angiopathy, Neonatal Hypoxic encephalopathy at UTHSC.
Education
- Bachelor's Degree
- Life Sciences and Bio-Informatics, ADU, India (2004-2007)
- Master's Degree
- Biotechnology, University of Ulster, United Kingdom (2007-2009)
- Doctorate Degree
- Gastrointestinal Microbiology, DlfE-Potsdam, Leibniz Institute, Germany. Mentor: Prof. Michael Blaut (2010-2014)
- Postdoctoral Training
- Department of Immunology and Pathology, Baylor College of Medicine, Houston, TX, USA. Mentor: Dr. James Versalovic (2014-2017)
- Postdoctoral Training
- Department of Neurology, UTHealth, Houston, TX, USA. Mentor: Dr. Louise McCullough (2017-2018)
Areas of Interest
Clinical Interests
Intrinsic connection between vascular diseases and exacerbation of gut inflammation
Research Interests
My research primarily focuses on exploring the intricate relationship between the microbiome and a range of health conditions, including stroke, hypertension, cerebral amyloid angiopathy, Alzheimer’s disease, and other age-related ailments. The overarching objective of my research endeavors is to shed light on how the intestinal microbiome influences the delicate balance of intestinal epithelial homeostasis, ultimately leading to the onset and exacerbation of chronic inflammation in the context of neurodegenerative diseases associated with aging.
Throughout my career, I have dedicated my efforts to investigating the role of gut microbiota in the pathogenesis of inflammatory diseases, particularly emphasizing intestinal inflammation. Furthermore, I harbor a deep interest in discerning ways in which the microbiota can be harnessed to yield beneficial health outcomes.
Presently, my research is laser-focused on the intriguing ” GUT-BRAIN Axis”. Our research team is delving into the intricate interplay between the microbiome and its secretory products, examining how these interactions impact the physiological processes of the brain, particularly in the context of age-related diseases. A notable aspect of our work involves the development of innovative germ-free rodent models, which serve as invaluable tools in unraveling the effects of individual or multiple known bacterial species and their secretions on the shaping of the intestinal epithelium and beyond.
My extensive utilization of germ-free mouse models has enabled me to investigate the consequences of both pathogenic and beneficial (probiotic) bacteria, further elucidating the multifaceted role of different bacteria in maintaining gut homeostasis. Additionally, I am conducting research into how the microbiome and its secretory products impact the physiology of goblet cells, specifically focusing on mucus synthesis. I aspire to identify changes in mucosal modifications, including immune responses, in relation to a dysbiotic gut microbiome, with the ultimate goal of identifying potential biomarkers for predicting age-related diseases at an earlier stage.
Publications
Ongoing Projects
Role of the gut dysfunction in central amyloid-β (Aβ) pathology and AD progression
The gut-brain axis (GBA) plays an increasingly recognized role in the central and cerebrovascular development at early stage in life. Especially some of the early gut microbiota colonizers like Lactobacillus and Bifidobacterium can synthesis neurotransmitters that can help improve memory as early as 3 months after birth. This they do by modulating the gut environment. We have a profound interest in understanding the role of gut microbiome (the population of living microbes that reside in the gastrointestinal tract) in modulation of host immune responses and in maintaining the gut barrier integrity. It is known that bacterial cell numbers outnumber our own cell numbers by a factor of 10. The gut microbiota is now considered an important partner to the host, interacting with virtually all mammalian cells. These microbes have been co-evolved with us and are capable of initiating independent signaling mechanisms in our bodies. The important roles of bacteria in host health and disease are still being explored. However, new studies show that under normal conditions there is a mutualistic relationship between the host and gut microbiota that play a key role in the maintenance of immunity, the preservation of epithelial barrier integrity and to the efficient absorption of nutrients in the gut. This mutualistic relationship can be compromised by shifts in the microbial composition, termed dysbiosis.
Dysbiosis in the gut has been shown to increase inflammatory disorders. Probiotics (beneficial microbes isolated from human breastmilk) has been used to treat the gut inflammation caused by various factors like hypoxia, pathogenic infection or immune dysfunction. Especially, changes in gut environment related to dementia has been studied recently. Since gut derived metabolites directly impact brain development, gut microbiota and gut physiology should be considered while developing new strategies to understand and treat diseases associated with dementia. Our lab believes that determining the appropriate balance of these intestinal microbial communities at various stages of life will help improve health and longevity. As a first step, examining the severity of gut inflammation, changes in gut microbiota and systemic immune changes at the pre-symptomatic time point. To do so we use various animal models (transgenic AD models, germfree models and infected models) that help us understand the communication between the gut microbiome, peripheral organs and the brain. Bacterial composition plays a vital role in maintaining the health of gut physiology and can be used as a therapeutic intervention to recover the gut.
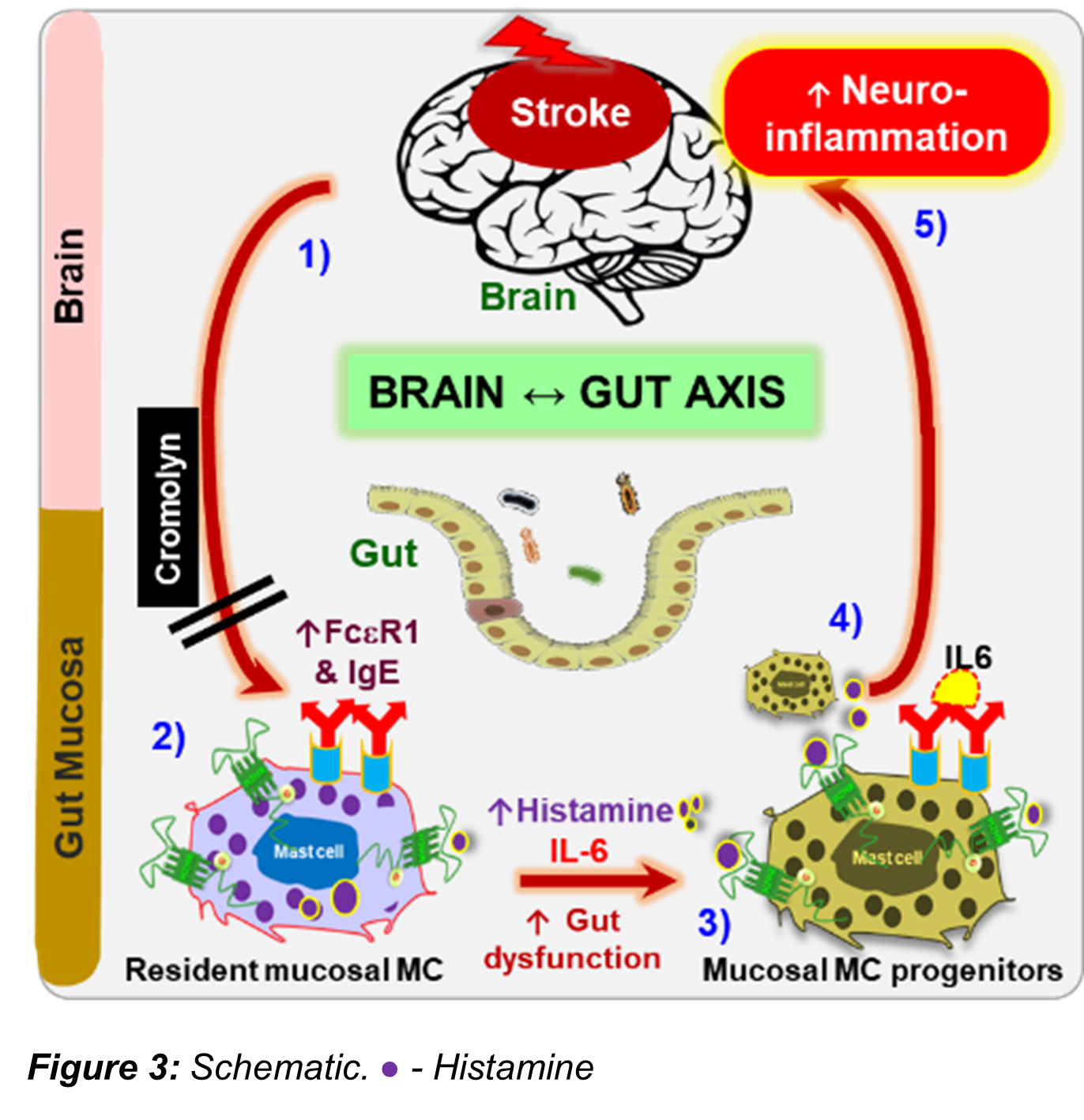
Mast cell connects the gut and the brain post-stroke
The importance of the bidirectional communication between the “gut-brain axis” in response to stroke is increasingly recognized. Many inflammatory processes are activated by ischemic stroke and contribute to brain injury and mortality especially in the elderly. Clinically, approximately 65% of stroke patients are left with functional impairments after stroke and 15% die shortly after their stroke. Increasing evidence suggests that peripheral inflammatory responses after stroke play an important role in determining neurological outcome. Mast cells (MCs) are one of the most rapid responders to injury. MCs release pre-stored and newly synthesized mediators specifically histamine (HA), a pro-inflammatory transmitter that enhances inflammation. Gut MCs are a major source of HA. MCs comprise 2–5% of the mononuclear cells in the normal gut mucosa and intestinal inflammation increases this number. Mucosal MC progenitors constitutively home to the intestinal mucosa and are recruited and mature during inflammation. HA is required for maturation of these MC progenitors. How stroke alters mucosal MC maturation in peripheral organs such as the gut, one of the major sources of HA releasing MCs, has not been explored. We propose that gut MCs are a major source of pro-inflammatory histamine after stroke. Our work has found that stroke elicits a vicious cycle of peripheral inflammation through communication within the brain-gut axis (BGA), leading to severe gut dysregulation, which is worse in aged animals. Aged MCs (MCs-Ag) are known to be in an increased state of activation at baseline. Thus, aging is a primary factor influencing the levels of HA. The study on gut MCs and its HA concentrations on post-stroke inflammation represents a novel therapeutic intervention that could be used to alter the gut milieu to improve post-stroke recovery.
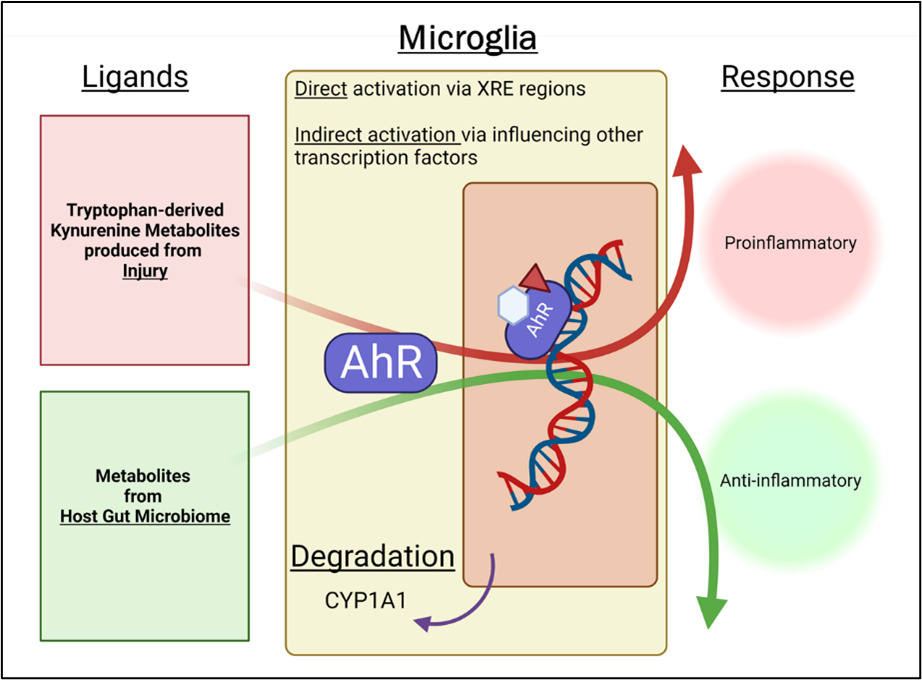
Impact of intestinal bacterial specific AHR ligands from the gut to the brain in aging and CAA
The contemporary understanding of age-related cognitive impairment centers on the interplay between neurodegenerative disease and cerebrovascular disease. A substantial body of literature suggests that cognitive impairment in the aging brain is driven by overlapping neurodegenerative and cerebrovascular pathologies. Cerebral Amyloid Angiopathy (CAA) is an emerging cause of vascular cognitive impairment (VCI). CAA is characterized by the deposition of toxic amyloid-β protein (Aβ) in the basement membrane of the cerebral vasculature and is the second most common cause of intracranial hemorrhage. The pathogenic pathways of CAA and Alzheimer’s Disease (AD) intersect at the levels of Aβ generation, its circulation and its clearance pathways, but diverge in their mechanisms of brain injury and disease presentation. Aging is the major risk factor for CAA. With aging, a disruption of gut microbiota homeostasis occurs, caused by an imbalance in the bacterial composition and their metabolic activity referred to as “dysbiosis”. Our data support the presence of gut dysbiosis in CAA animal models when compared to age-matched WT controls. This dysbiosis contributes to increased peripheral and central nervous system (CNS) inflammation, a hallmark of aging. Our lab has demonstrated that inducing dysbiosis in young mice by fecal microbiota transplant (FMT) from aged donors (FMT-Aged) increases neuroinflammation, cognitive deficits, and mortality. Gut dysbiosis induced peripheral immune modifications is a key mediator of the deleterious effects on microglial function in the brain, but the molecular mechanisms are unknown.
Cells continuously adapt to their milieu using specialized receptors. One of these sensors is the highly conserved ligand-activated transcription factor aryl hydrocarbon receptor (AHR) that integrates dietary, microbial, and metabolic cues to control the immune response. The regulatory role of AHR on inflammation is ligand-specific. On one hand, elevated plasma level of host-derived AHR ligands (e.g. L-kynurenine) are positively correlated with mortality in neuronal injury patients and inhibition of kynurenine-mediated activation of AHR by AHR inhibitors (AHRI) improves behavioral outcome. These studies suggest a detrimental role for AHR. On the other hand, AHR ligands derived from dietary tryptophan metabolism decrease microglial/astroglial activation during resolution of inflammation. These studies suggest a beneficial role for AHR when activated by microbiota-derived ligands (indole-derivatives) rather than host-derived ligands.