Sensitizing Tumors to RadiationTherapy Using Gold Nanoparticles

 Gold nanoparticles that accumulate passively within tumors have been shown to increase tumor sensitivity to radiation. But this approach generally requires very high concentrations of gold delivered intravenously, which is unaffordable, and low-energy X-rays that no longer are used clinically, according to radiation oncologist Sunil Krishnan, MD, professor and John P. and Kathrine G. McGovern Distinguished Chair in the Vivian L. Smith Department of Neurosurgery at McGovern Medical School at UTHealth Houston.
Gold nanoparticles that accumulate passively within tumors have been shown to increase tumor sensitivity to radiation. But this approach generally requires very high concentrations of gold delivered intravenously, which is unaffordable, and low-energy X-rays that no longer are used clinically, according to radiation oncologist Sunil Krishnan, MD, professor and John P. and Kathrine G. McGovern Distinguished Chair in the Vivian L. Smith Department of Neurosurgery at McGovern Medical School at UTHealth Houston.
In his laboratory at the Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, Krishnan and his research team are investigating the use of active targeting of tumors with small quantities of gold administered before a short course of radiation therapy with megavoltage X-rays from a linear accelerator.
“We decorate gold nanoparticles with peptides and antibodies that promote improved targeting of tumors, and when internalized they reside in close proximity to the DNA of cancer cells,” he says.
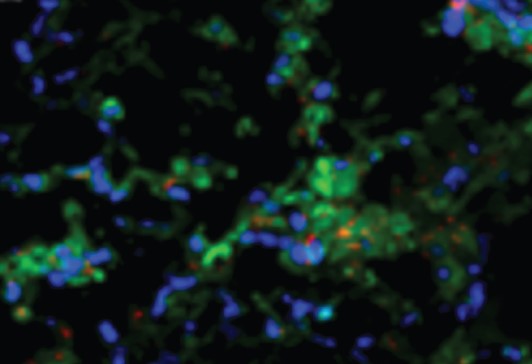
Dr. Krishnan’s team is
engineering nanoparticles
to penetrate desmoplastic
stroma of cancers. In the
photo below, red represents
gold nanorods, green shows
hypoxic cells, and blue
shows normoxic cells.
“Subsequent irradiation results in a more pronounced shower of secondary electrons, leading to oxidative stress and DNA damage to the tumor that amplifies the effect of the radiation therapy.”
Krishnan and his team of researchers have worked with gold nanoparticles for almost two decades. Their major thrust has been the design, fabrication, characterization, and validation of nanoparticles for the diagnosis and treatment of cancer.
“We began using them for hyperthermia – to warm up tumors – and segued to using them with radiation to enhance delivery,” he says. “The nanoparticles allow us to increase the dose of radiation the tumor receives without increasing the actual dose of radiation delivered.”
While his primary focus has been hepatobiliary, pancreatic, and rectal tumors, his direction is shifting to the use of gold nanoparticles in the treatment of brain tumors. “We’ve done work in the past to find ways to transport nanoparticles to tumors of the brain, but the brain is an especially challenging site because we have to deploy particles through the blood-brain barrier, a highly selective semipermeable border of endothelial cells which prevents substances in the blood from crossing into the extracellular fluid where neurons reside,” he says. “We’ve solved a lot of the issues related to transporting them from the injection site to the tumor, and we’re glad to be back in Houston focusing our energy on ways that allow gold nanoparticles to penetrate the blood-brain barrier.”
After completing residency training, Krishnan accepted his first faculty position at The University of Texas MD Anderson Cancer Center in 2004 and rose to full professor of radiation oncology, serving as director of the Center for Radiation Oncology Research and co-directing a T32 training grant on nanotechnology applications in cancer. In 2019, he joined Mayo Clinic in Florida as professor of radiation oncology, where he directed the Office of Clinical Trials for the institution and helped with research efforts across the department, institution, and cancer center.
He recently returned to Houston as professor of radiation oncology in the Vivian L. Smith Department of Neurosurgery. Krishnan’s research is funded with three R01 grants and a U01 from the National Institutes of Health.
Related Publications
Rauta PR, Mackeyev Y, Sanders K, Kim JBK, Gonzalez VV, Zahra Y, Shohayeb MA, Abousaida B, Vijay GV, Tezcan O, Derry P, Liopo AV, Zubarev ER, Carter R, Singh P, Krishnan S. Acidotic hypoxic pancreatic tumor microenvironment transforms gold nanorods into cell-penetrant particles for potent radiosensitization. Science Advances.
2022 11 Nov;8(45):eabm0729.
Raghuram S, Mackeyev Y, Symons J, Zahra Y, Gonzalez V, Mahadevan KK, Requejo KI, Liopo A, Derry P, Zubarev E, Sahin O, Kim JBK, Singh PK, Cho SH, Krishnan S. Uncloaking cell-impermeant gold nanorods via tumor microenvironmental cathepsin B facilitates cancer cell penetration and potent radiosensitization. Biomaterials. 2022
Nov 2;291:121887.
In This Section:
Features:
- True to Our Values
- The Heart-Brain Program: A Closed-Loop Continuum of Care to Patients with PFO-Associated Stroke
- Multidisciplinary Programs Provide Convenience and a Broader View of Health to Physicians and Patients
Patient Stories:
- Alabama Woman Finally Gets a Sleep Disorder Diagnosis Through Telehealth
- Radiofrequency Ablation (RFA) for severe back pain
- Finally Getting a Good Night’s Sleep with remedē®
- Making a Difference by Participating in Huntington’s Disease Research
Research & Trials:
- Sensitizing Tumors to Radiation Therapy Using Gold Nanoparticles
- An Investigation: Th-1 Dendritic Cell Immunotherapy in Combination with Standard Chemoradiation for Adjuvant Treatment of Adult Glioblastoma
- Advances in the Diagnosis and Management of Cluster Headache
- AI-Powered Algorithms May Help Detect Unruptured Brain Aneurysms
Accolades & News:
- Department of Neurosurgery Ranks 4th in NIH Funding Among US Clinical Science Departments
- McCullough Wins Stroke Association Lecture Award
- UTMOVE Receives Distinguished Edmond J. Safra Fellowship in Movement Disorders
- Schiess Awarded UTHealth Houston President’s Scholar Award
- Burish Co-Chairs the AHS Scientific Program Committee
- Furr Stimming Named HSG Outstanding Investigator
- Lo Receives UT System STARs Award
- Burish Assumes Responsibility for Neurology Residency Training for Headache Medicine
- Morcos Named Chair of Neurosurgery