Clinical Research & Trials
In the field of Pediatric Infectious Diseases, research is crucial for preventing, diagnosing, and treating infections. The Division of Pediatrics Infectious Diseases boasts a strong history of groundbreaking, high-impact clinical research with more than 25 years of experience in clinical trials across all phases. The research team partners with different sponsors, academic networks, and investigators to develop these products from discovery to market. These discoveries play a vital role in generating evidence for optimal treatment and diagnosis of infectious diseases in children.
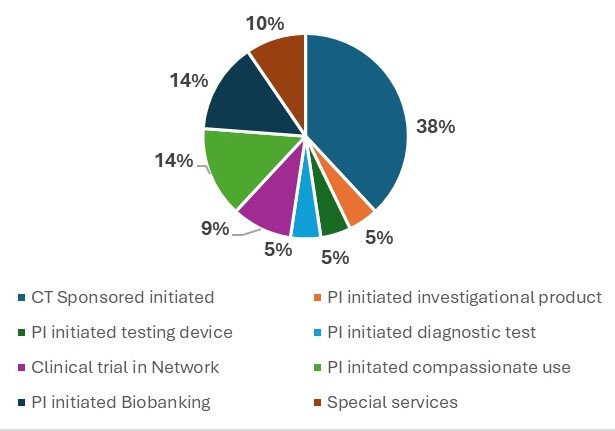
This graph gives a general breakdown of pediatric infectious diseases annual clinical research.
The Division participates in diverse interdisciplinary research projects exploring exciting topics in infectious disease, including antibiotic resistance, emerging infections, and the use of safe and effective vaccines. This collaboration with a variety of entities — sponsors, contract research organizations, the U.S. Food and Drug Administration (FDA), the UTHealth Houston institutional review board, and clinical sites — has grounded the team’s expertise in practical application.
Current enrolling studies are related to determining the safety and effectiveness of medications, vaccines, devices, diagnostic products, and treatment regimens intended for babies, children, and adolescents. For many studies, UTHealth Houston is among the five top enroller sites in the USA with outstanding performance in data collection, vaccine administration, and safety follow-up.
The Infectious Diseases Division is also extremely active in industry-sponsored clinical trials. Some of the conditions studied include virological suppression, human immunodeficiency viruses (HIV), gram-negative bacterial infections, and more. At any given time, the Division has trials in active start-up and enrollment with companies such as GlaxoSmithKline, Pfizer, Astra Zeneca, Sanofi, and many more.