Clinical Trials
Designed to test the safety and effectiveness of treatments, clinical research depends on the participation of volunteers with HD. Participants are typically divided into at least two groups, usually randomly: one that receives the treatment and one that does not.
Clinical trials are time-limited, so participants can join, or enroll, in the studies only when they are recruiting. Historically, clinical trials for Huntington’s disease have only included people who are gene positive and symptomatic. Due to findings from some recent research, those who are gene positive and not yet fully symptomatic, or are early in the disease, are now also being included in HD clinical trials.
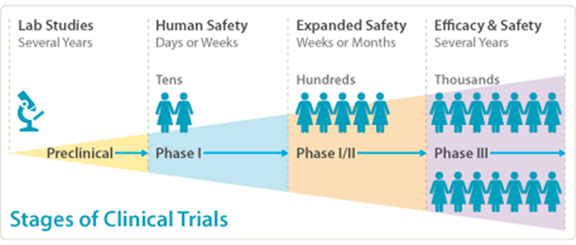
Stages of Clinical Trials
Learn more about clinical research from the Huntington’s Study Group.
https://neurosciences.ucsd.edu/centers-programs/huntingtons-disease/
research/clinical-observational-trials.html
Phase I
The goals of this first step in testing a new treatment or drug in humans are to make sure the treatment is safe, and to figure out the right dose to use in future studies. In this phase, researchers mainly watch for side effects rather than how well the drug works against a disease. They also look at how the body handles the drug, that is, how a drug is absorbed, distributed, metabolized, and excreted by the human body. This is done by taking regular blood samples to measure how much of the drug is in the blood. Phase I studies are typically done with healthy volunteers, but sometimes people with the disease are included – especially if the drug only works in those with a specific condition or if it is a combined Phase I/Phase II study.
Phase II
This is the second step in testing a new drug or treatment in people. Now that the drug has passed basic safety checks in Phase I, researchers want to see if it actually works for the condition it is meant to treat. They also continue to look at side effects and fine-tune the dose. These studies usually involve more participants than Phase I—often hundreds—and the people in the trial have the disease or condition the drug is meant to treat. Sometimes the new drug is compared to a placebo (a dummy treatment) or to a standard treatment to see how well it performs.
Phase III
This is the final big test before a new drug can be approved for general use. By now, the drug has been shown to be safe and to work in smaller groups of people (from Phase I and II), but now researchers want to make sure it works on a larger scale. These studies involve a lot more people, often thousands, and usually test the drug in different real-world settings. The goal is to compare the new drug against a commonly used drug, and to keep an eye out for any side effects.