First infusion in Houston for new Alzheimer’s drug administered at UTHealth Houston
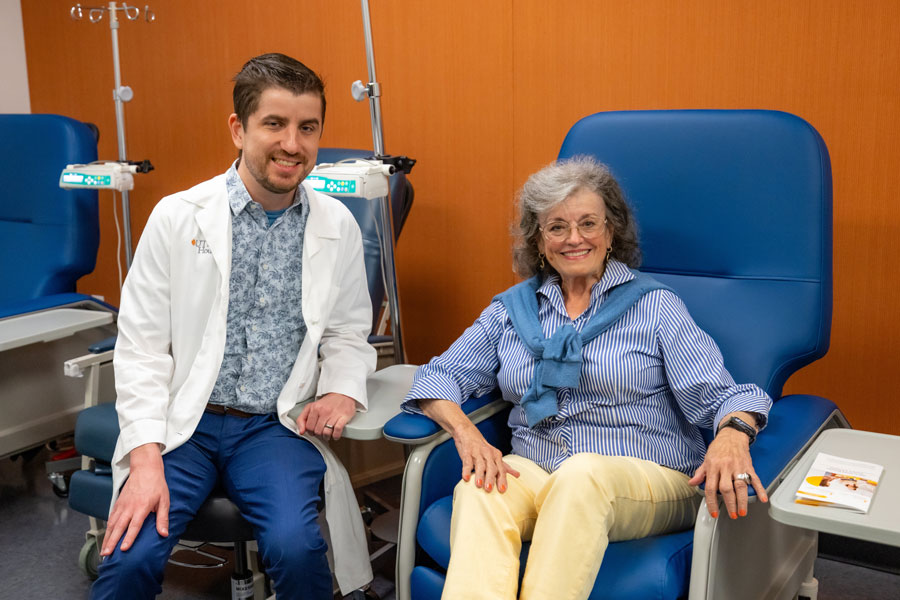
UTHealth Houston is the first institution in Houston to administer an FDA-approved drug, Kisunla (donanemab-azbt), for the treatment of early symptomatic Alzheimer’s disease.
The recipient was 79-year-old Terrie Frankel.
“We’re making history today,” Frankel said.
Frankel began experiencing memory loss and forgetfulness at the beginning of this year. After consulting Rehal Bhojani, MD, primary care and sports medicine physician with McGovern Medical School, she was referred to David Hunter, MD, associate professor in the Department of Neurology with McGovern Medical School.
Kisunla isn’t a cure for the disease, but clinical trial results showed it reduced amyloid plaques on average by 84% at 10 months after infusion – appearing to slow the progression of Alzheimer’s for those in the early stages. UTHealth Houston was one of the sites in the study.
“Mrs. Frankel is the ideal patient for this treatment,” Hunter said. “We want to see patients as soon as they, or their family, notice the slightest trace of forgetfulness. The earlier the patient is in their Alzheimer’s disease, the more they benefit from treatments like Kisunla.”
Frankel will receive monthly Kisunla infusions for the next 18 months. She will also undergo MRIs after each of the first several infusions to monitor for possible side effects.
Frankel will also have frequent PET scans to check how the amyloid plaque is reacting to the treatment.
“If we notice it is gone, we will reduce the frequency of infusions to once every six months,” Hunter said.
Paul E. Schulz, MD, professor of neurology and director of the UTHealth Houston Neurosciences Neurocognitive Disorders Center with McGovern Medical School, led the clinical trial for Kisunla at UTHealth Houston.
“For the first time in human history we now have two drugs that significantly slow the course of Alzheimer’s disease,” Schulz said. “Having two drugs is way better than one because now we know that the approach to the disease is generally correct. Up until recently, we were wondering whether we were barking up the wrong tree after having a lot of negative studies using similar approaches. But now we have a lot of confidence that we’re on the right pathway to treatments for Alzheimer’s disease. Alzheimer’s is a very emotionally distressing disease, so anything we do to slow its course has a big impact on a lot of patients and families.”
UTHealth Houston is involved in several Alzheimer’s disease trials. Starting in 2025, researchers will be recruiting participants over the age of 55 with a family history of dementia, but no memory loss themselves.
“We can also be very proud of everybody here at UTHealth Houston and all of our patients that have been involved in these trials that are having a positive outcome now. It’s become a very emotionally rewarding field in which to be working now that we can finally offer people things that we know work, while at the same time we are continuing to look for additional medications to improve outcomes even further,” said Schulz, who is the Rick McCord Professor in Neurology and the Umphrey Family Professor in Neurodegenerative Diseases at McGovern Medical School.
“My hope for being part of this is that it will be a notable treatment for other folks and that there is hope for those who recognize their symptoms early,” Frankel said.