Wu research on novel microglia cells published in Immunity

New research from the lab of Long-Jun Wu, PhD, professor at the Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, discovering a novel subpopulation of brain immune cells has been published in Immunity.
The paper, “Rod-shaped microglia interact with neuronal dendrites to attenuate cortical excitability during TDP-43-related neurodegeneration,” was led by first author Manling Xie, PhD, and Wu, the senior author.
The researchers discovered a distinct subpopulation of microglia, the brain’s primary immune cells, that may play a protective role in amyotrophic lateral sclerosis. Unlike typical, ramified, branching form of microglia, these newly identified microglia are elongated and align themselves along neurons during disease progression. This transformation appears to regulate nerve cell activity and represents a natural protective response in the brain.
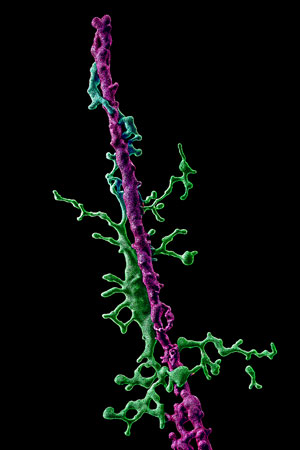
To study this process, the team combined multichannel recordings with two-photon in vivo imaging to measure neuronal activity. High-resolution microscopy of brain tissue, including samples from individuals with ALS, confirmed the presence of rod-shaped microglia and their close interaction with neurons.
Additionally, the researchers used spatial and single-cell RNA sequencing to generate detailed transcriptional profiles and applied genetic and molecular approaches to study how specific genes, such as TREM2, influence microglial behavior.
“We found that rod-shaped microglia play an active role in protecting neurons in neurodegeneration,” said Wu, the founding director of the IMM Center for Neuroimmunology and Glial Biology and the C. Harold and Lorine G. Wallace Distinguished University Chair at McGovern Medical School at UTHealth Houston. “By understanding this process, we may uncover new therapeutic targets in ALS and potentially other neurodegenerative diseases.”
Moving forward, the team will begin investigating the molecular mechanisms that drive the formation of rod-shaped microglia, identifying unique molecular markers that could serve as therapeutic targets, and exploring the signaling pathways that prompt these cells to interact with neuronal dendrites.
“This work bridges immunology and neuroscience, showing how the brain’s resident immune cells directly control neuron activity in the context of neurodegeneration,” Wu said.
Wu thanked his team, particularly Yue Liang, PhD, for her help with key experiments during the revision. Dennis Dickson, MD, at the Mayo Clinic, made important contributions demonstrating the remarkably similar rod-shaped microglia in ALS patient brain samples. The team also received support and resources from the Genome Analysis Core and the Brain Bank for Neurodegenerative Disorders at the Mayo Clinic.