How to Begin Research Projects at UTH: Practical Tips and Resources
Fellowship Research Orientation 2024-25
By: Efstratios Koutroumpakis, MD, FACC
Outline
- Milestones/expectations/timeline
- Research events throughout the academic year
- Examples of recent ongoing or completed research projects
- Available Resources at UT and MDA
- Research project opportunities
Fellowship Research Milestones & Expectations
PGY4 fellows
- First semester:
- Identify mentor(s) and have a first meeting
- Identify at least one research area/topic of interest
- Second semester:
- Provide title of project and description in PICO format.
- Identify research team and appropriate resources, barriers. Develop a realistic/achievable research plan.
- Obtain IRB approval and fulfill any administrative requirements to start study.
- Start preparing for presentation in October of next year/preliminary analysis.
PGY5 fellows
- First semester:
- Start and probably complete data collection
- Identify a journal target for publication or conference target for abstract submission
- Second semester:
- Finalize data collection
- Data analysis and interpretation
PGY6 fellows
- First semester:
- Present findings at a national meeting
- Draft manuscript
- Prepare poster presentation for research retreat
- Second semester:
- Finalize manuscript
- Submit for publication
- Prepare Powerpoint presentation for graduation
Research events throughout the academic year
| October 18th, 2024 | March 20th, 2025 | May, 2025 |
|---|---|---|
| PGY5 fellow research presentations | 5:00-7:00 PM Research Retreat Cooley Center |
End-of-year presentations |
Website: https://med.uth.edu/internalmedicine/cardiovascular-medicine/education/crf/
National conferences for 2024-25
| ASCNC 2024 | September 5-6, 2024 Austin, TX |
| TCT | October 27-30, 2024 Washington, DC |
| AHA Scientific Sessions 2024 | November 16–18, 2024 Chicago, IL |
| ACC.25 | March 29 – 31, 2025 Chicago, IL |
| SCAI 2025 Scientific Sessions | May 1 – 3, 2025 Washington, DC |
| SCCT 2025 | July 17-20, 2025, Montreal Canada |
| ASE 2025 | September 5-7, 2025 Nashville, TN |
Examples of Ongoing Research Projects and Mentors
| Brief Title | Mentor |
| Effect of radiotherapy on cardiac implantable electronic devices | Karimzad |
| Effects on energy drinks on health | Higgins |
| Atrial fibrillation anticoagulation prescription practices between Harris Health and UT | Chiadika |
| Value of left atrial strain in patients with cryptogenic stroke | Ekeruo |
| Impact of IR/Met-syn on diastolic dysfunction | Prakash |
| Impact of COVID on Cardiovascular Health Access Disparities amongst Patients with Cancer and Cardio-Oncology Disease | Iliescu |
| Prognostic value of left atrial stain in AL amyloidosis and its predictive value in determining development of atrial fibrillation | Koutroumpakis |
| NASA Cardiac imaging and coronary disease in space flight | Bungo |
| Diastolic Dysfunction in Pregnancy: Echocardiographic Parameters in Patients with Cardiovascular Comorbidities. | Tung |
| Steroid use in cardiac transplant patients | Patarroyo |
Examples of recent abstracts at national meetings and publications

How to Begin Research Projects at UTH: Practical Tips and Resources
By: Siddharth Prakash, MD, PhD
Proper Management of HIPAA Data
Drop Box
Unencrypted email
Unencrypted text
Google Drive
Thumb Drive
OneDrive
(Encrypt) Email
PerfectServe
Epic messages
VPN
UTH Server
Websites to Know
- Share documents with HIPAA data (OneDrive)
- Submit and modify research protocols
- Required human subjects research training
Center for Clinical Research and Evidence-Based Medicine:
Research Consultations
Steps in Launching a Research Project:
- Idea
- Detailed Literature Review
- Study Question
- Detailed Study Protocol
- Funding (if needed)
- Approval from Physicians, IRB, Hospital
- Implementation
Center for Clinical Research and Evidence-Based Medicine:
Research Consultations
Stating the Questions/Hypothesis:
- A research idea (for an observational or intervention study) should be structured into a well-built clinical research question or hypothesis with the following PICO components:
- Population of interest
- Intervention to be tested
- Comparison strategy
- Outcome(s)
Components of a Clinical Research Protocol
- Inclusion and Exclusion Criteria
- Recruitment Methods
- Procedures for group assignments and/or interventions (if prospective)
- Management of confounding variables
- Primary and secondary outcomes with methods of assessment
- Analysis plan
- Limitations
- References
New for 2023! Design and Reporting Guidelines Required by Journals
- STROBE or RECORD checklist for observational studies
- PRISMA checklist for systematic reviews
- Many journals will not publish without documentation that data was collected appropriately
- Know before you start so that you are not limited
STROBE checklist: for case-control and cross-sectional studies
A TMC Librarian Can Help to Formulate Research Questions and Do Literature Searches
Consult With a TMC Librarian | https://askus.library.tmc.edu/
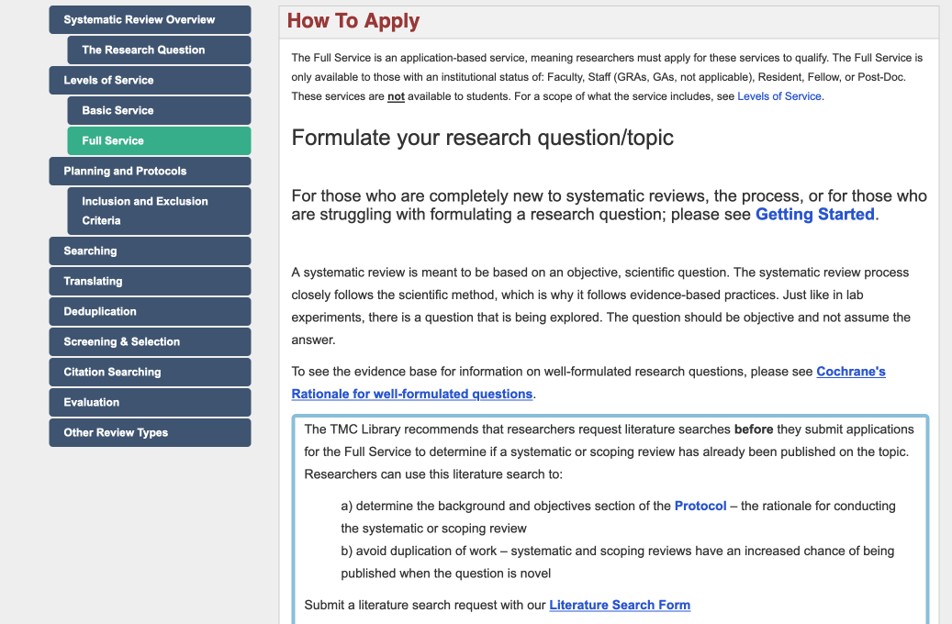
Committee for the Protection of Human Subjects:
Protocol Workflow (tips on starting research in slideset)

UTH Research Resources
- Center for Clinical Research and Evidence-Based Medicine
- McGovern Medical School Information Technology (MSIT)
- McWilliams School of Biomedical Informatics
- School of Public Health
- Texas Advanced Computing Center (TACC)
- Committee for the Protection of Human Subjects (CPHS)
- Your fellowship research committee!
Center for Clinical Research and Evidence-Based Medicine:
Research Consultations
SPARK mentoring and consultations on designing and conducting clinical and translational studies
Contact person: Maureen Goode, PhD, ELS, [email protected] – 713-500-7924
The mission of SPARK is to mentor junior investigators and assist experienced investigators by providing multidisciplinary assistance on the design, implementation, and conduct of clinical and translational research studies.
SPARK services include:
- Guidance on the formulation of research projects and programs
- Advice on protocol design
- Assistance with IRB/CCTS applications
- Suggestions on finding outside funding
- Research career advice
Center for Clinical Research and Evidence-Based Medicine:

https://med.uth.edu/crebm/clinical-research-education/clinical-research-curriculum/
Contact: Deborah Garcia | (713) 500-6708
School of Medical Bioinformatics:
Clinical Data Warehouse
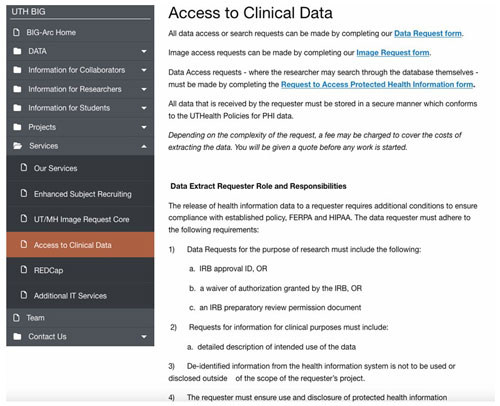
https://redcap.uth.tmc.edu/surveys/?s=79RDHRDPLH
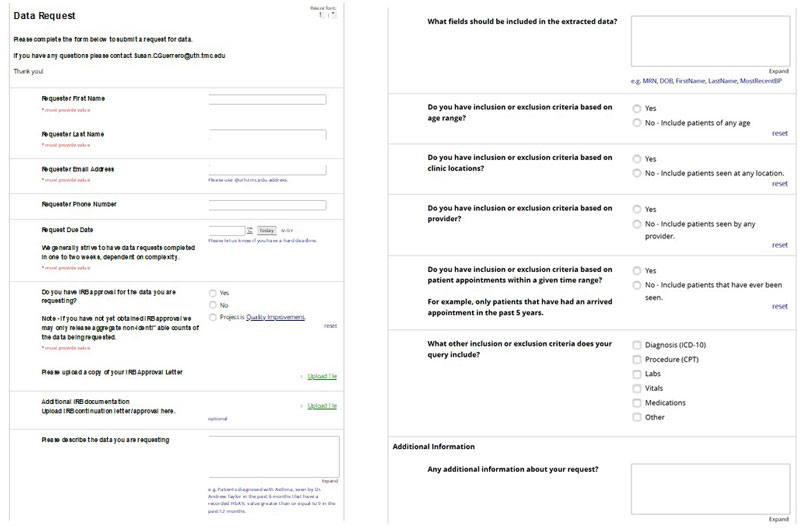
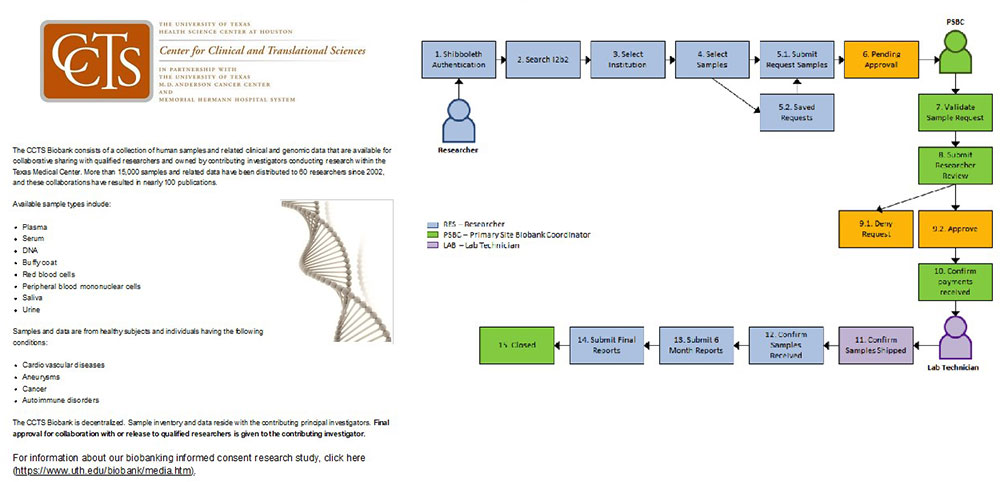
Center for Clinical and Translational Sciences:
Biobank
School of Public Health: Center for HealthCare Data Available Datasets
https://sph.uth.edu/research/centers/center-for-health-care-data/
School of Public Health: Center for HealthCare Data
How to Apply
New for 2023! Fellow Research website: https://med.uth.edu/internalmedicine/cardiovascular-medicine/education/crf/

New for 2023! Review scientific manuscripts
- Cardiology faculty receive manuscripts to review on a daily basis
- We do not have time to review them all
- Reviewing for journals is something you can put on your CV
- Builds academic currency
- Develop experience in your field
- If you want to be offered a chance to review manuscripts, email: [email protected]
- Include your subspecialty interest(s)
- Note: reviews generally need to be completed within 1-2 weeks
New for 2023! Fellow education in Cardiovascular Genomics
- NIH-funded UTH online course to educate clinicians about CV genetic diseases: HCM, Long QT, Aortic dissections, etc.
- 24 modules, each take 10-20 minutes to complete
- Bring yourself up to speed on genomic medicine
- Get a certificate to put on your CV

https://go.uth.edu/CVGenomicsCert
Research Opportunities: Siddharth Prakash
- Heritable aortopathies: noninvasive assessment of aortic hemodynamics (central arterial waveforms, pulse wave velocity, 4DMRI)
- Role of exercise as a therapy for thoracic aortic disease
- Clinic cohort: outcomes of watchful waiting period for patients with large aneurysms
- GenTAC and Montalcino Aortic Consortium registries: genotype-phenotype correlations with bicuspid aortic valve
- Turner Syndrome Research Registry: natural history of Turner syndrome registry participants with thoracic aortic aneurysms
MDA Research Resources
- EHR Analytics & Reporting Team – assistance with data collection and analysis via different tools e.g. SlicerDicer, Universe
- REDCap through MD Anderson for data storage
- Biostatistical support (biostatistician of Cardiology Department at MDA)
- Manuscript/grant editing services, Research Medical Library at MDA: https://mdandersonorg.sharepoint.com/sites/research-medical-library/SitePages/Our-Editing-Services.aspx
- For further instructions on accessing the above resources, please reach out to Stratos Koutroumpakis ([email protected])
Research Opportunities: Efstratios Koutroumpakis
- Cardiac AL Amyloidosis: development of imaging biomarkers for early diagnosis, risk stratification and treatment response.
- Radiation-induced carotid artery disease (accelerated atherosclerosis/baroreflex failure): early detection and evaluation of preventive therapies.
- Development of cardiometabolic disease in patients with prostate cancer treated with hormone therapy
- Chemotherapy-induced cardiomyopathy in adolescents and young adults with sarcomas/lymphomas; early detection and risk mitigation
Research Opportunities: Poyee Tung
- Cardio-Obstetrics:
- Outcomes of pregnant patients with LVEF <45% at UT practice.
- Outcomes of patients sent for advanced heart failure therapies.
Harmancey lab | Cardiometabolic Research
Approach
Our lab employs a wide array of imaging and molecular techniques to study the pathophysiology of heart failure at multiple levels, including in isolated cells, isolated working hearts, and whole model organisms.
Our expertise includes
- Modeling of metabolic and heart diseases in small animal models
- Assessment of cardiac metabolism/work relationship
- Manipulation of gene expression in vitro and in vivo
- Protein signaling and gene expression network analysis at tissue and single cell levels
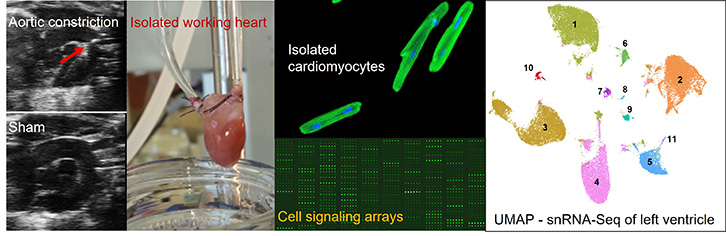
Current and emerging research projects:
- Investigating the role of the Nuclear Receptor NR4A2 in cardiac remodeling during injury and failure.
- Deciphering the intracellular signaling pathways and physiological functions of candidate olfactory receptors in the normal and diseased heart.
Contacts: [email protected] | [email protected]

Research Opportunities: Jean-Bernard Durand
- Cardio-Oncology:
- Evaluation of Cardiac Reserve by Dobutamine Stress Echocardiography among Patients with Cancer and Cardiomyopathy.