Discovery of molecular mechanisms required for memory formation
Over the past few decades, a large body of evidence has shown that the transcription factor CREB plays an obligatory role in different types of memory. The original findings implicating CREB in memory was discovered when I was a post-doctoral fellow in the laboratory of Dr. Eric Kandel. We have continued to identify the various components of the signaling pathways that lead to CREB activation and increased expression of CREB-responsive genes that are critical for long-term memory formation (Figure 1). In addition, our group was the first to demonstrate that mTORC1, which is activated by growth factor and energy signals, is required for long-term memory (Figure 2). We have also been examining the role of cortical plasticity in memory consolidation, and have discovered that the activation of plasticity-related signaling occurs in both the neocortex and hippocampus immediately after training (Figure 3). These findings challenged the long-held belief that plasticity occurs first in the hippocampus and is later transferred to the neocortex. Recently, we have been carrying out multi-electrode recordings to examine the development of plasticity in the prefrontal cortex in response to behavioral training. An appreciation of these mechanisms is critical for understanding the basis of memory impairments seen after brain trauma and as a result of normal aging.
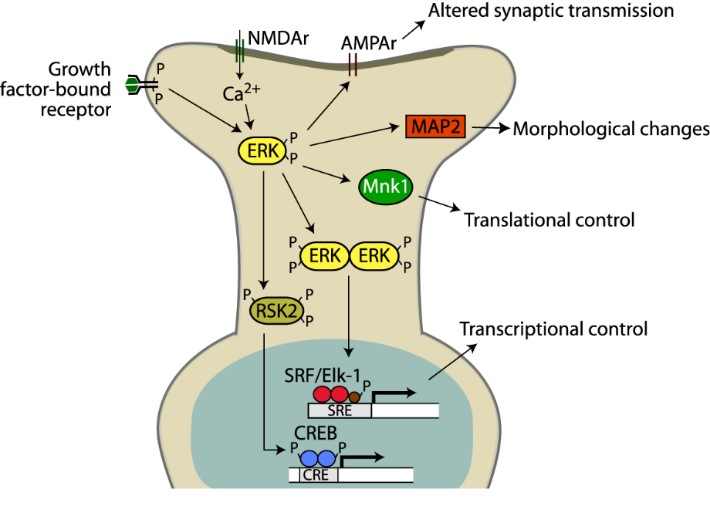 Figure 1. Long-term neuronal plasticity is regulated by a series of intracellular signaling pathways. Unlike short-term memory, long-term memory requires the de novo expression of proteins required for morphological changes of neurons. Our laboratory has identified several obligatory components of how behavioral training leads to altered gene expression and synaptogenesis.
Figure 1. Long-term neuronal plasticity is regulated by a series of intracellular signaling pathways. Unlike short-term memory, long-term memory requires the de novo expression of proteins required for morphological changes of neurons. Our laboratory has identified several obligatory components of how behavioral training leads to altered gene expression and synaptogenesis.
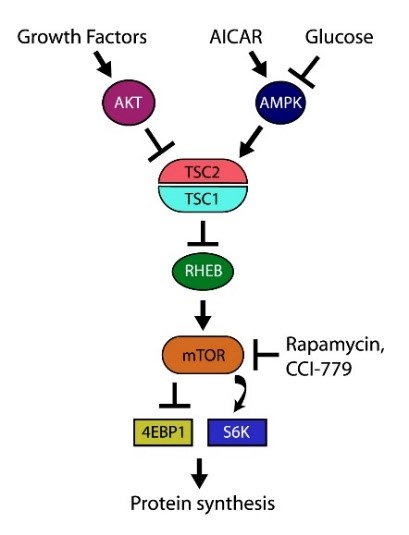 Figure 2. Growth factor and energy signals are key regulators of protein synthesis. Our research has shown that the ability of growth factors and glucose to enhance long-term memory are dependent on the activation of the mTORC1 pathway. Associated with this, we have found that the learning and memory dysfunction that accompanies diseases such as Tuberous Sclerosis (which leads to chronic overactivation of mTORC1) can be alleviated by drugs such as Rapamycin.
Figure 2. Growth factor and energy signals are key regulators of protein synthesis. Our research has shown that the ability of growth factors and glucose to enhance long-term memory are dependent on the activation of the mTORC1 pathway. Associated with this, we have found that the learning and memory dysfunction that accompanies diseases such as Tuberous Sclerosis (which leads to chronic overactivation of mTORC1) can be alleviated by drugs such as Rapamycin.
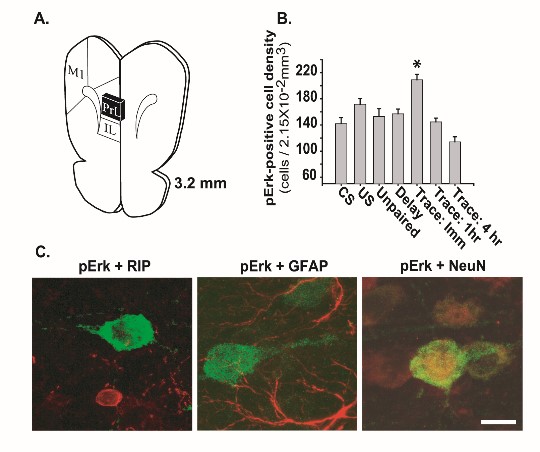 Figure 3. It has been proposed that long-term memory is critically dependent on the function of the hippocampus, and that over time, memories become hippocampal independent as they are “transferred” to the neocortex. Using a behavioral task that engages both the prefrontal cortex and the hippocampus, we have found that plasticity-related signaling (e.g. phosphorylation of Erk) occurs in both structures immediately after training. These and other findings have led to a re-envisioning of the role of the hippocampus in consolidating declarative memories.
Figure 3. It has been proposed that long-term memory is critically dependent on the function of the hippocampus, and that over time, memories become hippocampal independent as they are “transferred” to the neocortex. Using a behavioral task that engages both the prefrontal cortex and the hippocampus, we have found that plasticity-related signaling (e.g. phosphorylation of Erk) occurs in both structures immediately after training. These and other findings have led to a re-envisioning of the role of the hippocampus in consolidating declarative memories.
Selected reading:
- Dash, P.K., Hochner, B., and Kandel, E.R.: Injection of cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature, 345:718-721, 1990.
- Dash, P.K., Karl, K.A., Colicos, M.A., Prywes, R., and Kandel, E.R. (1991) CRE-binding protein is activated by Ca2+/calmodulin as well as cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. 88:5061-5065, 1991.
- Blum, S., Moore, A.N., Adams, F.S., and Dash, P.K.: A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J. Neurosci., 19:3535-3544, 1999.
- Hebert, A.E., and Dash, P.K.: Extracellular signal-regulated kinase activity in the entorhinal cortex is necessary for long-term spatial memory. Learn Mem, 9:156-166, 2002.
- Runyan, J., Moore, A.N. and Dash, P.K. A role for prefrontal cortex in memory storage for trace fear conditioning. J. Neurosci, 24:1288-1295, 2004.
- Runyan, J. and Dash, P.K. Intra-medial prefrontal administration of SCH-23390 attenuates Erk phosphorylation and long-term memory for trace fear conditioning in rats. Neurobiol Learning and Memory 82:65-70, 2004.
- Dash P.K., Hebert A.E., and Runyan J.M. A unified theory for cellular and systems memory consolidation. Brain Res Rev, 45:30-37, 2004.
- Hebert A.E. and Dash P.K. Non-redundant roles for hippocampal and entorhinal cortical plasticity in spatial memory storage. Pharm Bioch and Behav, 79:143-153, 2004.
- Dash, P.K., Orsi, S.A. and Moore, A.N. Spatial memory formation and memory-enhancing effect of glucose involves activation of the tuberous sclerosis complex-Mammalian target of rapamycin pathway. J Neurosci. 26:8048-8056, 2006.
- Way SW, Rozas NS, Wu HC, McKenna J 3rd, Reith RM, Hashmi SS, Dash PK and Gambello MJ. The differential effects of prenatal and/or postnatal rapamycin on neurodevelopment defects and cognition in neuroglial mouse model of tuberous sclerosis complex. Hum Mol Genet, 21:3226-36, 2012.
- Rozas NS, Redell JB, Pita-Almenara J, McKenna J, Moore AN, Gambello MJ and Dash PK. Intrahippocampal glutamine administration inhibits mTORC1 signaling and impairs long-term memory. Learning and Memory, 22:239-246, 2015.
- Fischer TD, Dash PK, Liu J, Waxham MN. Morphology of mitochondria in spatially restricted axons revealed by cryo-electron tomography. PLoS Biol. 16:e2006169, 2018.
- Hylin MJ, Zhao J, Tangavelou K, Rozas NS, Hood KN, MacGowan JS, Moore AN, Dash PK. A role for autophagy in long-term spatial memory formation in male rodents. J Neurosci Res. 96:416-426, 2018.