Researchers Seek to Improve Outcomes in Children with Malignant Fourth Ventricular Brain Tumors Through Novel Studies
Only a very small amount of the chemotherapeutic drugs given systemically for the treatment of pediatric brain tumors actually reaches the brain, due to the blood-brain barrier’s efficiency at excluding the entry of most agents that circulate in the blood. As a result, the outlook for children with recurrent malignant brain tumors is extremely poor. Most clinical trials offer systemic chemotherapy or radiation therapy, both of which have side effects and often fail in children with recurrent tumors. To address this issue, David I. Sandberg, MD, FAANS, FACP, FAAP, is leading two single-center studies at McGovern Medical School at UTHealth Houston and Children’s Memorial Hermann Hospital. Both investigate novel therapies with the potential to improve outcomes for children with fourth ventricular brain tumors, while avoiding systemic toxicity.
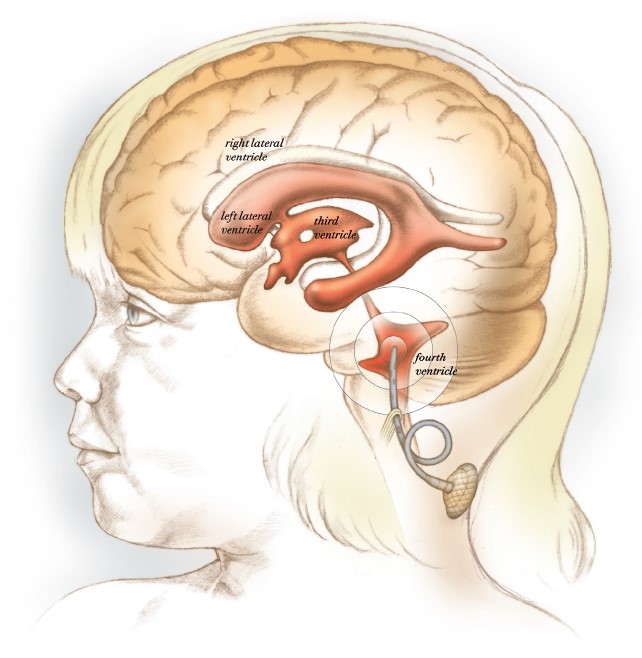
In a clinical trial led by Sandberg, chemotherapeutic drugs are infused into the brain’s fourth ventricle to avoid systemic toxicity.
“Despite the advances we’ve made in pediatric neuro-oncology, we’re still seeing far too many children die of malignant brain tumors. We believe we can do better,” says Sandberg, professor and director of pediatric neurosurgery, the Dr. Marnie Rose Professor in Pediatric Neurosurgery, and THINK Neurology Chair in Pediatric Tumor Research and Innovation in the Department of Pediatric Surgery and Vivian L. Smith Department of Neurosurgery at McGovern Medical School.
Infusion of Panobinostat (MTX110) into the Fourth Ventricle or Tumor Resection Cavity in Children and Adults with Recurrent Medulloblastoma: A Pilot Study
According to the American Cancer Society, about 500 children are diagnosed every year with medulloblastoma, the most common malignant brain tumor in children. Current treatments expose children to considerable toxicity, and when tumors reoccur despite treatment, survival rates are low. Sandberg is hopeful that a novel clinical trial of MTX110, a new formulation of soluble panobinostat from Midatech Pharma, will help patients overcome the devastating disease.
The clinical trial follows a successful study he led in an animal model, which demonstrated that MTX110 can be safely infused in the fourth ventricle and can achieve drug levels dramatically higher than intravenous or oral administration of the same drug. The team at McGovern Medical School found no neurological deficits after fourth-ventricle infusions in the preclinical study.
“Our objective was to test the safety and pharmacokinetics of short-term and long-term infusions of MTX110, a chemotherapeutic agent that inhibits the growth of medulloblastoma,” Sandberg says. “In the animal study group there were no MRI signal changes in the brainstem, cerebellum, or elsewhere in the brain. In addition, the cytoarchitecture of the brain was preserved in all of the animals, with only mild postsurgical changes.
“We are really excited about the promising data from these experiments,” says Sandberg, who is lead author of an article detailing results in the Journal of Neurosurgery: Pediatrics.1 The pilot study, which has been approved by the U.S. Food and Drug Administration, will enroll five patients with recurrent medulloblastoma.
For more information, visit clinicaltrials.gov/ct2/show/NCT04315064.
Combination Intraventricular Chemotherapy Pilot Study: 5-Azacytidine (5-AZA) and Trastuzumab Infusions into the Fourth Ventricle or Resection Cavity in Children and Adults with Recurrent or Residual Posterior Fossa Ependymoma
Sandberg considers ependymoma a significant target for the fourth-ventricular infusion approach, as standard chemotherapy drugs almost invariably are ineffective. “Standard chemotherapy given orally or intravenously has almost no impact on the majority of kids with recurrent ependymoma,” he says. “These tumors
are relentless.”
According to the American Cancer Society, about 2,000 children and adults in the United States are diagnosed with ependymoma each year. The overall five-year survival rate is 83.9%. “We’ve had preliminary success with a drug called 5-azacytidine, which caused shrinkage of some of the tumors we treated in our last trial, but it wasn’t enough. It didn’t cure children. So we’ve added a second drug called trastuzumab, which is active against ependymoma. We’re hoping that the combination of these drugs will have an even greater impact,” he says.
Patients who enroll in the trial will receive weekly 10-milligram intraventricular 5-AZA infusions and weekly 21-milligram intraventricular trastuzumab infusions for six consecutive weeks. All patients will undergo an MRI of the brain and total spine within seven days after the final 5-AZA and trastuzumab infusion to determine their response to the treatment and to assess for any signal changes in the brain or spine caused by the infusions. Additionally, there will be 30-day and 90-day follow-up assessments by telephone or in person.
Enrollment is limited to 10 study participants. To be eligible, participants must be between 1 and 80 years old and must have histologically verified ependymoma that originated in the back of the head, with recurrence or progression anywhere in the brain and/or spine. Patients are also eligible if they have a residual tumor that has not been completely cleared despite prior treatments.
To learn more, visit clinicaltrials.gov/ct2/show/NCT04958486.
For more information about enrolling in either of the two clinical trials, contact Bangning Yu, RN, PhD, at bangning.yu@uth.tmc.edu or call (713) 500-7363.
1Sandberg CI, Kharas N, Yu B, Janssen CF, Trimble A, Ballester LY, Patel R, Mohammad AS, Elmquist WF, Sirianni RW. High-dose MTX110 (soluble panobinostat) safely administered into the fourth ventricle in a nonhuman primate model. J Neurosurg Pediatr. 2020 May 1:1-9.
Inside this Edition:
- Responsive Neurostimulation for Generalized Epilepsy in an 18-Year-Old
- The NICU Follow-Up Clinic: Everything Premature Babies Need To Get the Best Start in Life
- Team-Based Medicine Leads to Nonsurgical Repair of Depressed Skull Fractures in Two Neonates
- Watchful Waiting for Cerebral Venous Thrombosis Allows a Boy To Avoid Brain Surgery
- UTHealth Houston Team Completes Feasibility Study for Minimally Invasive Myelomeningocele Repair in 25 Patients
- Developing Better Laboratory Models of Neonatal Intraventricular Hemorrhage and Hydrocephalus
- Researchers Seek to Improve Outcomes in Children with Malignant Fourth Ventricular Brain Tumors Through Novel Studies
- McGovern Medical School Alumni Blend Neurology Expertise and Philanthropy To Advance Pediatric Tumor Research