Colon Bundle Protocol for Pediatric Surgical Patients
This project was led by Dr. Justin Lee from Phoenix Children’s and Dr. Elizabeth Fialkowski from Doernbecher Children’s and is now retired.
Building on research from the OHSU and WPSRC that demonstrated improved clinical outcomes following the implementation of a colon bundle protocol, similar colon bundle protocol will be implemented across participating PSQC institutions. [1,2] Consensus views on feasibility, published literature, and guidelines will be used to formulate a NSQIP appropriate colon bundle protocol. Using the NSQUIP data collection and risk-adjusted outcomes-based approach, this study seeks to understand the effects of such protocol on postoperative recovery and postoperative outcomes. Participation in this pilot study can help identify areas of improvement, reduce variations in practice, and measure clinical outcomes.
Project Overview:
This project builds on the bundle approach study2 which demonstrated a reduction in post-op SSIs in adult colorectal surgery patients through the use of a prescribed treatment bundle which targets pre-op, intra-op and post-op pathways and details the best utilization of antibiotics, the preparation of the surgical site, and instrument tray guidance around surgical wound closure. This project has been piloted in the Western Pediatric Surgery Research Consortium and demonstrated a positive effect.
This project’s objective is to substantially reduce the incidence of SSIs post operatively for our pediatric patients undergoing colorectal procedures with an anastomosis and abdominal closure through the use of a standard procedure checklist.
The project has a well-defined list of procedures for inclusion and a procedure bundle for standardization (below). Compliance to the checklist will be classified as low (1-4 bundle elements completed); high (5-8 bundle elements completed); or ideal (all bundle elements completed). Our balancing measure will be no increase in the rate of SSIs or readmissions in this patient group. Ultimately qualitative interviews may be conducted near project conclusion to collect best practices from good performers and barriers from less good performers. These qualitative findings will be collated into an implementation guide and broadly distributed. Project duration is estimated to be 18-24 months.
Data Collection Requirements and Storage:
The project workgroup will create processes for which variables to collect. Several are included in current NSQIP-P SARs. These are all GI mortality, morbidity, and SSIs (superficial, deep and organ space) filtered by the cpt inclusion criteria. Additional variables may include ED revisit, 30-day readmissions, unplanned return to OR, anastomotic leak and bundle compliance. Education will be provided around how to build custom variables into the NSQIP platform; mechanisms within various EMR platforms to embed the checklist; how to download the Data Download Report (DDR) into an Excel spreadsheet with the correct variables selected and all PHI scrubbed; and how to save the report in the NSQIP workstation for ease of future abstraction.
Checklist:

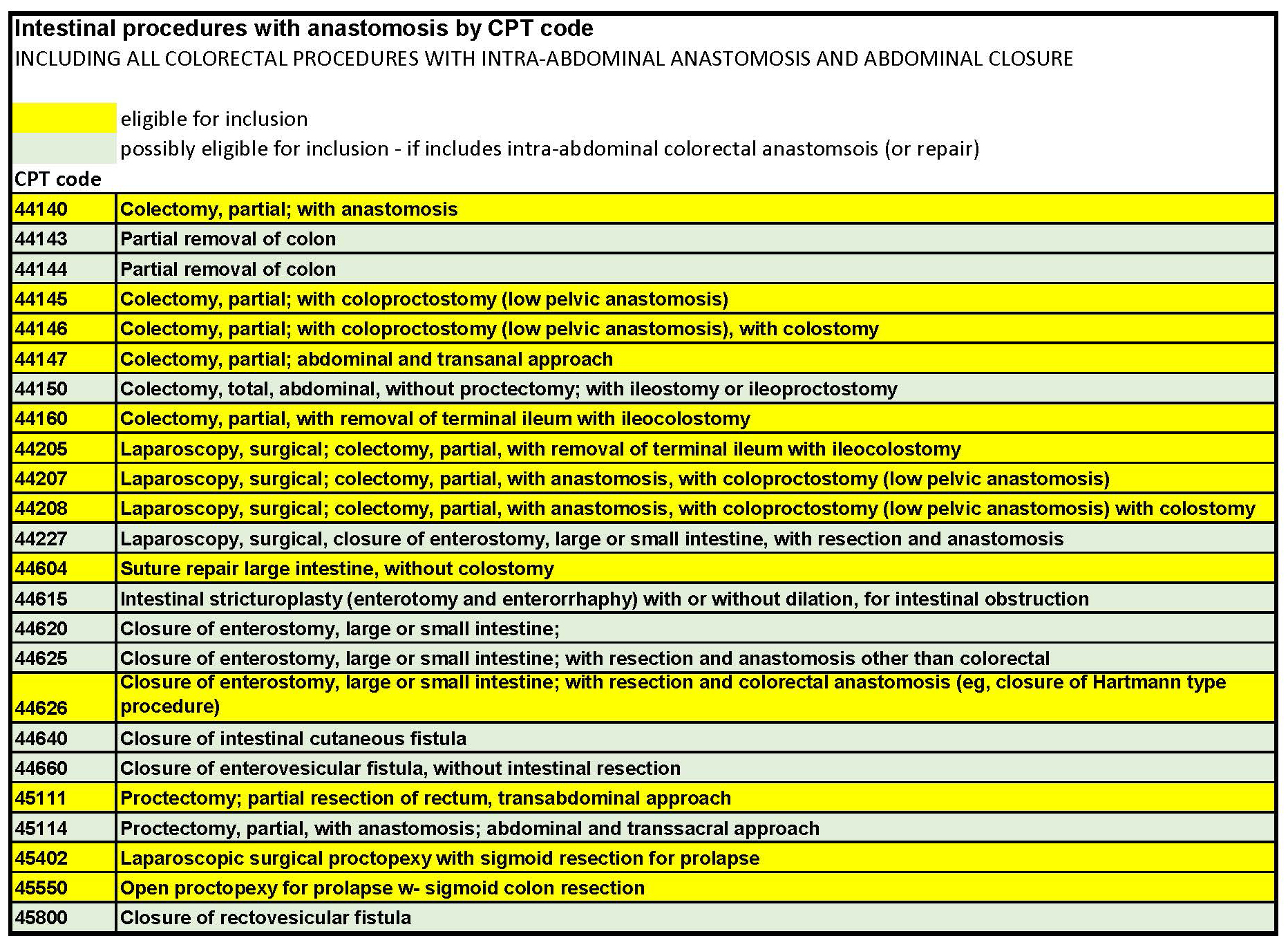
CPTs for case Inclusion:
SCR Documents*:
- Custom Variable Creation Guide
- Data Submission Guide
- Operations Manual
*Please contact the PSQC Program Manager, Terry Fisher, for the PDF copy.
Workgroup Members:
Dr. Brian Coakley, Pediatric Surgeon, Mount Sinai Kravis Children’s, NY,NY
Dr. Jennifer DeFazio, Pediatric Surgeon, New York-Presbyterian Morgan Stanley Children’s, NY,NY
Dr. Elizabeth Fialkowski, Pediatric Surgeon, Doernbecher Children’s, Portland, OR
Ms. Tara Foster, SCR, Albany Medical Center, Albany, NY
Dr. Mike Goretsky, Pediatric Surgeon, King’s Daughters’ Children’s, Norfolk, VA
Dr. Akemi Kawaguchi, Pediatric Surgeon, Children’s Memorial Hermann, Houston, TX
Dr. Justin Lee, Pediatric Surgeon, Phoenix Children’s, Phoenix, AZ
Ms. Lori Montgomery, SCR, Cook Children’s, Fort Worth, TX
Dr. Elizabeth Renaud, Pediatric Surgeon, Hasbro Children’s, Providence, RI
Christine Velazco, Pediatric Surgeon, Arnold Palmer Children’s, Orlando, FL
For additional information, please contact the PSQC Program Manager, Terry Fisher.
Return to PSQC Homepage