Stem Cell Research
Traumatic injuries lead to significant morbidity and mortality, particularly among children, adolescents, and young adults. More specifically, traumatic brain injury (TBI) contributes to half of all trauma-related deaths and frequent, devastating prolonged morbidity, imposing a huge economic impact on individuals and society. There is no current treatment to reverse the cellular destruction associated with TBI. Cellular therapy is a burgeoning field of experimental treatment that has shown promise in the management of many diseases, including TBI. The overarching objective of our clinical, translational, and basic science program is to explore the therapeutic potential of cellular therapy for traumatic brain injury.
We use a controlled cortical impact (CCI) injury rat model of TBI. After cellular injection, the rats are tested for improvements in motor and cognitive function. Cellular changes in the rat brain are then identified through magnetic resonance imaging and immunohistochemistry. We have the ability to isolate and characterize various stem and progenitor cell populations, including rat mononuclear cells (rMNC), rat mesenchymal stem cells (rMSC), rat hematopoietic stem cells (rHSC), and rat multipotent adult progenitor cells (rMAPC). In addition, we collaborate with other labs to obtain rat neural stem cells (rNSC) and human mesenchymal stem cells (hMSC).
Rat Mesenchymal Stem Cells
Detailed Immunophenotype characterization
Proinflammatory state
Examination of the acute, local proinflammatory state after TBI
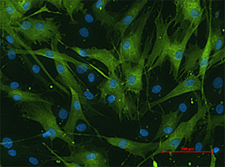
MSC’s from GFP+transgenic rodents
Investigation of the limitations of isolation and expansion
We have numerous specific areas of ongoing investigation. Overall, we hope to gain insight into the therapeutic potential of the various stem cell populations mentioned above. In addition, we hope to better characterize the optimal route(s) of administration, as well as optimal cell number. More specifically, given the increasing evidence that cellular death continues over a significant period of time, we are studying delayed cellular therapies for TBI. Most traumatically injured patients suffer concomitant injuries, so we will study the differential migration of cells to various organs in a “poly-trauma” model (combining TBI with hemorrhagic shock, pulmonary contusion, etc). In order to increase our understanding of processes at the cellular level, we will continue to better characterize our cells through flow cytometry and immunocytology. We also study the microenvironment interactions between transplanted and native cells, including co-culture work and incubation of cells with TBI extract.
– Charles S. Cox Jr. MD
Stem Cell Therapy
Safety of Autologous Stem Cell Treatment
Traumatic Brain Injury in Children
A phase I clinical trial
We initiated a phase I clinical trial in 2006, evaluating the use of autologous bone marrow derived mononuclear cells to treat children with isolated severe traumatic brain injury (TBI). The study has completed enrollment of 10 patients in early 2009. The trial is registered on www.clinicaltrials.gov and performed under FDA IND RB 12620. This is a collaborative effort between leading institutions in the Texas Medical Center including The University of Texas Medical School at Houston, Memorial Hermann Children’s Hospital, MD Anderson Children’s Cancer Hospital, and The Center for Cell and Gene Therapy (CAGT) at Baylor College of Medicine. Pediatric patients (age 5-14) with a post-resuscitation Glascow Coma Score (GCS) between 5 and 8 are identified and further evaluated. Inclusion criteria also include consent within 24 hours of injury and the ability of child and caretaker to speak English (necessary for future neuropsychiatric testing). After consent is obtained, cells are obtained via bone marrow aspiration (3 ml/kg of body weight). The mononuclear cell fraction is isolated at the CAGT and 6 million cells/kg are then infused intravenously.
The safety of the therapy is determined by monitoring cerebral and systemic hemodynamics during harvest and infusion, neurologic events (seizures, change in GCS, CVA), infectious morbidity, and secondary organ injury (particularly pulmonary and hepatic). Late outcomes are determined using Glasgow Outcomes Scale Scores (GOS), and a battery of functional outcome measures. This phase I trial will answer the question of safety with intravenous administration of autologous bone marrow cells. Additionally, comparison of this small cohort with matched controls may offer limited insight into the efficacy of cell therapy for TBI and will open the door for larger clinical trials investigating optimal treatment strategy (cell number, cell type, delivery timing, delivery route, etc) and overall efficacy. This study was presented at the Congress of Neurological Surgeons in New Orleans in October, 2009.
Phase I Clinical Trials in the immediate investigative pipeline include:
Autologous, human umbilical cord blood, mononuclear cell infusions for the sub-acute treatment of TBI.
Pre-clinical studies are investigating the role of heterologous cellular therapies for TBI.