Brain channels ‘stopped in time’ reveal chemical flow that enables learning, thinking
 In an effort to understand how brain cells exchange chemical messages, scientists say they have successfully used a highly specialized microscope to capture more precise details of how one of the most common signaling molecules, glutamate, opens a channel and allows a flood of charged particles to enter. The finding, which resulted from a study led by Johns Hopkins Medicine researchers in collaboration with McGovern Medical School investigators, could advance the development of new drugs that block or open such signaling channels to treat conditions as varied as epilepsy and some intellectual disorders.
In an effort to understand how brain cells exchange chemical messages, scientists say they have successfully used a highly specialized microscope to capture more precise details of how one of the most common signaling molecules, glutamate, opens a channel and allows a flood of charged particles to enter. The finding, which resulted from a study led by Johns Hopkins Medicine researchers in collaboration with McGovern Medical School investigators, could advance the development of new drugs that block or open such signaling channels to treat conditions as varied as epilepsy and some intellectual disorders.
A report on the experiments, funded by the National Institutes of Health, was published March 26 in the journal Nature. Elisa Carrillo, PhD, assistant professor of biochemistry and molecular biology, is second author, and Vasanthi Jayaraman, professor and chair ad interim of the Department of Biochemistry and Molecular Biology, is the co-corresponding author.
“Neurons are the cellular foundation of the brain, and the ability to experience our environment and learn depends on [chemical] communications between neurons,” said Edward Twomey, PhD, assistant professor of biophysics and biophysical chemistry at the Johns Hopkins University School of Medicine.
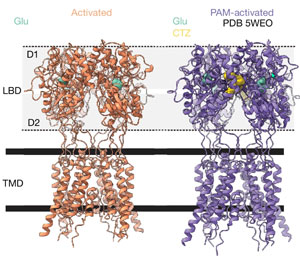
Scientists have long known that a major molecule responsible for neuron-to-neuron communications is the neurotransmitter glutamate, a molecule abundant in the spaces between neurons. Its landing place on neurons is a channel called an AMPA receptor, which interacts with glutamate, and then acts like a pore that takes in charged particles. The ebb and flow of charged particles creates electrical signals that form communications between neurons.
To figure out details of the miniscule movements of AMPA receptors (at the level of single atoms), researchers used a cryo-electron microscope (cryo-EM) to image these channels during specific steps in the communications processes.
Typically, scientists find it easier to study cell samples that are chilled, a state that provides a stable environment. But at normal body temperature, Twomey’s team found that the AMPA receptors and glutamate activity increased, providing more opportunities to capture this process in cryoEM images.
“We were excited to be a part of this team doing the functional studies. We were able to show that increasing the temperature led to an increase in activation of these proteins. This is paradigm shifting as until now most functional and structural studies performed at room temperature showed the protein to be primarily inactive in the prolonged presence of glutamate,” said Jayaraman, who also holds the John S Dunn Professorship and Bob and Hazel Casey Endowed Chair.
To that end, the scientists purified AMPA receptors, taken from lab-grown human embryonic cells that are used widely in neuroscience research to produce such proteins. Then, they heated the receptors to body temperature (37 degrees Celsius or 98.6 degrees Fahrenheit) before exposing them to glutamate. Immediately after this, the receptors were flash frozen and analyzed with cryoEM to get a snapshot of the AMPA receptors bound to the major signaling molecule, glutamate.
After assembling more than a million images taken with cryoEM, the team found that glutamate molecules act like a key that unlocks the door to the channel, enabling it to open more widely. This occurs by the clamshell-like structure of the AMPA receptor closing around glutamate, an action that pulls open the channel below.
Twomey said the findings could be used to develop new drugs that bind to AMPA receptors in different ways that either open or close the signaling channels of brain cells.
“With each new finding, we are figuring out each of the building blocks that enable our brains to function,” Twomey said.
Anish Kumar Mondal, from Johns Hopkins, also contributed to the research.
Research funding was provided by the National Institutes of Health (R35GM154904, R35GM122528), the Searle Scholars Program and the Diana Helis Henry Medical Research Foundation.