Deep Brain Stimulation Program
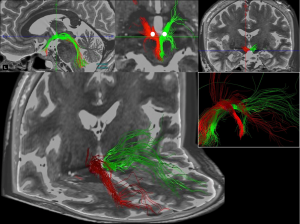 The Deep Brain Stimulation (DBS) surgery program focuses on delivering optimal treatment to patients suffering from movement disorders such as Parkinson’s disease, tremor, and dystonia, as well as refining treatments for patients with refractory psychiatric disease states, such as treatment resistant depression. In the majority of these surgeries, we record from the subcortical structures of the awake patient in an effort to better understand the disease process, refine the targeting, and together with new brain imaging techniques provide exemplar outcomes. Thus, the operating room is our chief laboratory space, to both learn from each patient and optimize treatment strategies.
The Deep Brain Stimulation (DBS) surgery program focuses on delivering optimal treatment to patients suffering from movement disorders such as Parkinson’s disease, tremor, and dystonia, as well as refining treatments for patients with refractory psychiatric disease states, such as treatment resistant depression. In the majority of these surgeries, we record from the subcortical structures of the awake patient in an effort to better understand the disease process, refine the targeting, and together with new brain imaging techniques provide exemplar outcomes. Thus, the operating room is our chief laboratory space, to both learn from each patient and optimize treatment strategies.
Outside the operating room, the use of imaging to illustrate both the structural and functional connectivity of the networks involved in various pathophysiologies treated by deep brain stimulation can improve our understanding of the disease process and potentially lead to more strategic treatment paradigms.
Simultaneously with learning intraoperatively from human research subjects, animal models of disease enable us to understand the biochemical mediators of such pathological cortical states and how the intervention of deep brain stimulation improves aberrant subcortical firing to improve symptomatology.
SELECTED PUBLICATIONS:
Fenoy AJ, Schulz P, Selvaraj S, Burrows C, Spiker D, Cao B, Zunta-Soares G, Gajwani P, Quevedo J, Soares J. Deep brain stimulation of the medial forebrain bundle: Distinctive responses in resistant depression. J Affect Disord. 203:143- 151, 2016
Fenoy AJ, McHenry MA, Schiess MC. Speech changes induced by deep brain stimulation of the subthalamic nucleus in Parkinson disease: involvement of the dentato-rubro-thalamic tract. J Neurosurg. 2016 Sep 9:1-11.
Fenoy AJ, Schiess MC. Deep Brain Stimulation of the Dentato-Rubro-Thalamic tract: Outcomes of Direct Targeting for Tremor. Neuromodulation. 2017. DOI: 10.1111/ner.12585.
Dandekar MP, Luse D, Hoffmann C, Cotton P, Peery T, Ruiz C, Hussey C, Giridharan VV, Soares JC, Quevedo J, Fenoy AJ. Increased dopamine receptor expression and anti-depressant response following deep brain stimulation of the medial forebrain bundle. J Affect Disord. 2017 Apr 5;217:80-88. DOI: 10.1016/j.jad.2017.03.074.
Fenoy AJ, Schulz P, Selvaraj S, Burrows C, Zunta-Soares G, Durkin K, Zanotti-Fregonara P, Quevedo J, Soares J. A longitudinal study on deep brain stimulation of the medial forebrain bundle for treatment-resistant depression. Transl Psychiatry. 2018 Jun 4; 8(1):111. doi: 10.1038/s41398-018-0160-4.
Fenoy AJ, Schiess MC. Comparison of Tractography-Assisted to Atlas-Based Targeting for Deep Brain Stimulation in Essential Tremor. Mov Disord. 2018; 33(12):1895-1901. DOI: 10.1002/mds.27463.
Dandekar MP, Saxena A, Scaini G, Shin JH, Migut A, Giridharan VV, Zhou Y, Barichello T, Soares JC, Quevedo J, Fenoy AJ. Medial Forebrain Bundle Deep Brain Stimulation Reverses Anhedonic-Like Behavior in a Chronic Model of Depression: Importance of BDNF and Inflammatory Cytokines. Molecular Neurobiology. 2019; 56(6): 4364-4380.