The Krishnan Nanotechnology and Radiation Biology Laboratory

Sunil Krishnan, MD, is a radiation oncologist at McGovern Medical School and UT Health Science Center Houston with a laboratory research program based primarily in the Institute of Molecular Medicine within the Center for Translational Cancer Research and partly in the Medical School Building Extension in the Department of Neurosurgery.
His laboratory research focuses on strategies to (i) sensitize tumors to radiation therapy using nanoparticles, chemotherapy, botanicals, immunotherapy, and novel radiation techniques and (ii) protect normal tissues from radiation injury using novel agents. His clinical and translational research focuses on studying the role of radiation therapy in the management of gastrointestinal cancers and identifying molecular and radiographic biomarkers that predict treatment response.
A major thrust of the laboratory has been the design, fabrication, characterization, validation, and mechanistic inquiries of nanoparticles for diagnosis and treatment of cancer. These include carbon-based, organic, inorganic, hybrid, and multilayered nanostructures.
Pivotal work has focused on the use of gold nanoparticles as radiation sensitizers via radiation dose enhancement and/or photothermal activation, and designer nanoparticles that improve pharmacokinetics and payload delivery characteristics via stimulus-responsiveness, bio-inspired molecular mimicry, Trojan-horse approaches, and phagocytosis evasion strategies.
The laboratory also anchors a Center for Physical Energy Therapeutics where we evaluate new strategies of combining localized physical energy therapeutics (ionizing and non-ionizing radiation of all flavors, thermal therapies, ultrasound) with targeted therapeutics (custom nanoparticles, antibody-drug conjugates, targeted protein degraders, immunotherapeutics) in a one-two punch synthetical lethality approach.
Current Projects
Sensitizing tumors to radiation therapy using gold nanoparticles
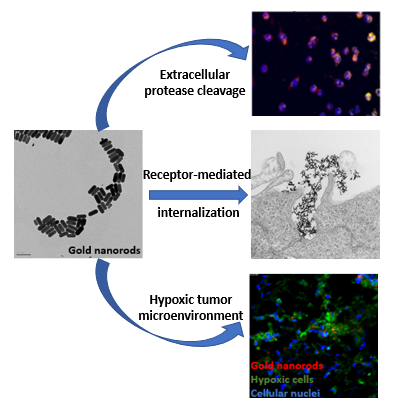 Gold nanoparticles (GNPs) accumulating passively within tumors have the potential for significant radiosensitization via an increase in secondary electron showers emanating from the highly electron dense (high atomic number) gold struck by ionizing radiation. This approach, however, generally requires low energy kilovoltage x-rays (not clinically utilized any longer), very high concentrations of gold administered intravenously (not clinically feasible or affordable), and immediate irradiation after gold administration (not easily implemented in a clinical workflow).
Gold nanoparticles (GNPs) accumulating passively within tumors have the potential for significant radiosensitization via an increase in secondary electron showers emanating from the highly electron dense (high atomic number) gold struck by ionizing radiation. This approach, however, generally requires low energy kilovoltage x-rays (not clinically utilized any longer), very high concentrations of gold administered intravenously (not clinically feasible or affordable), and immediate irradiation after gold administration (not easily implemented in a clinical workflow).
Our approach based on active, instead of passive, targeting shows a promising outlook for clinical translation in the near term given its reliance on small quantities of gold administered once before a short course of radiation therapy with megavoltage x-rays. By decorating GNPs with peptides and antibodies that promote tumor-homing and intracellular internalization, these GNPs are deployed within the cytoplasm/nucleus in close proximity to the DNA of the cancer cell; subsequent irradiation results in oxidative stress and DNA damage that amplifies the effect of radiation given by itself.
Strategies being studied to improve this approach include schemes to (i) ferry more GNPs to the tumor without entrapment in the liver/spleen via customized coating with stealth epilayers or extrinsic triggering of payload release from Trojan horses or actuation of cell penetrance based on intrinsic tumor properties like hypoxia/extracellular proteases, (ii) disperse intracellular clumps of GNPs, (iii) simultaneously abrogate antioxidant response pathways, and (iv) target cancer-specific and immune-resistance pathways concurrently. Unique tools utilized in these studies include UV-Vis spectrophotometry, infrared spectroscopy, dynamic light scatter, zeta potentiometry, x-ray diffraction analysis, high purification liquid chromatography, mass spectrometry, inductively coupled plasma mass spectrometry, thermogravimetry, lyophilization, microscopy (transmission/scanning electron, bright/dark field, hyperspectral, epifluorescence, confocal, multiphoton, high-resolution, and intravital), and flow cytometry.
Sensitizing pancreatic tumors to radiation therapy using molecular targeted agents
 There is often a disconnect between the preclinical promise of combining radiation therapy (RT) with molecular targeted therapies and the clinical failure of such combination therapy. This is, in part, driven by preclinical studies that are often restricted to combination of targeted agent and RT alone and not the standard-of-care curative-intent chemoradiation therapy (CRT), insufficient preclinical data on optimal sequencing of therapies, inappropriate animal models, inadequate understanding of mechanistic interactions, lack of clinical trial enrichment with predicted responders based on biomarkers, and lack of toxicity data.
There is often a disconnect between the preclinical promise of combining radiation therapy (RT) with molecular targeted therapies and the clinical failure of such combination therapy. This is, in part, driven by preclinical studies that are often restricted to combination of targeted agent and RT alone and not the standard-of-care curative-intent chemoradiation therapy (CRT), insufficient preclinical data on optimal sequencing of therapies, inappropriate animal models, inadequate understanding of mechanistic interactions, lack of clinical trial enrichment with predicted responders based on biomarkers, and lack of toxicity data.
To overcome these limitations of past approaches, we utilize a three-step approach to identify potential synthetic combinations. First, we use a novel high-throughput screen for replicative cell death that incorporates CRT and targeted agents and then validate promising agents in standard-format in vitro clonogenic assays. Next, we test promising agents in heterotopic xenograft cancer models in immune-deficient mice and in autochthonous orthotopic cancer models in immune-competent mice. Lastly, we use functional assays and novel in silico screens performed in parallel to serve as discovery tools for mechanisms of action and biomarkers of sensitivity or resistance.
We are one of five NIH-funded radiation biology laboratories nationally that evaluates CTEP drugs as putative radiation sensitizers in an effort to accelerate the advancement to the clinic of lead compounds that have been fully vetted in preclinical studies closely mimicking clinical treatment scenarios. Unique tools utilized in interrogating tumor heterogeneity in these studies include single cell sequencing, single cell proteomics, lipidomics, metabolomics, flow cytometry, SILAC, CyTOF, digital spatial profiling (transcriptome and proteome), reverse-phase protein array, and imaging mass cytometry.
Physical energy therapeutics
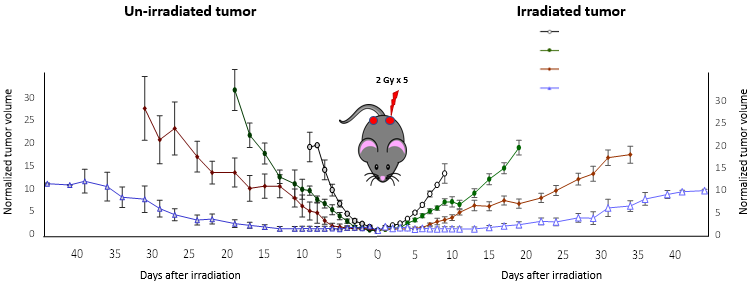
 We propose a one-two punch strategy whereby a dose of extrinsic energy is used to focally target tumors stereotactically or physically and then a second punch entails targeted therapeutics that home onto prosurvival pathways that are triggered only within tumor cells by this focal physical therapy.
We propose a one-two punch strategy whereby a dose of extrinsic energy is used to focally target tumors stereotactically or physically and then a second punch entails targeted therapeutics that home onto prosurvival pathways that are triggered only within tumor cells by this focal physical therapy.
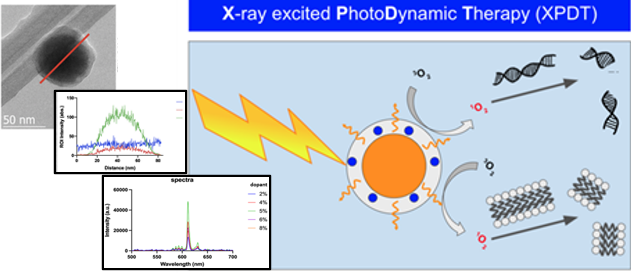
In one such iteration, we irradiate tumors with the most intense part of a proton beam (near its distal edge) and note increased expression of immune markers on the tumor cell surface. When appropriately coupled with immunotherapy targeting this marker, a robust anti-tumor response is elicited in not only the irradiated tumor but also the unirradiated tumor at a distant site. In another approach we have identified a similar immune-mediated regression of distant tumors by localized photodynamic therapy of tumors coupled with immunotherapy.
However, in a departure from using poorly penetrant light to trigger photodynamic therapy we use deep penetrating x-rays to trigger nano-sized bioinert phosphors that scintillate and activate a photosensitizer on their surface. This approach opens the door to other therapeutics that are hobbled by the limited penetration depth of light such as novel molecular nanomachines triggered by light to drill holes into cancer cells.
Lastly, we are methodically identifying tumor cell-specific markers of insult by sublethal doses of radiation therapy that can then be targeted using antibody-drug conjugates for synthetic lethality in a one-two punch approach. This approach uses radiation to paint a bulls-eye on the tumor and a heat-seeking drone/missile to eliminate it. Unique tools utilized in these studies include RNA sequencing, proteomics, lipidomics, metabolomics, flow cytometry, antibody drug conjugation, targeted protein destruction methodologies, and phage display.
Radiation protection
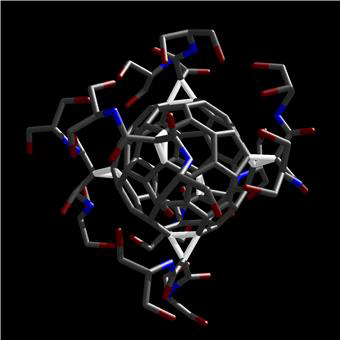 Widening the therapeutic window of radiation therapy can be achieved either via sensitizing the tumor to radiation therapy or protecting the surrounding normal tissue from radiation injury (or both). The laboratory is investigating the use of [60]fullerene derivatives that scavenge free radicals as potential radioprotectors and/or radiomitigators.
Widening the therapeutic window of radiation therapy can be achieved either via sensitizing the tumor to radiation therapy or protecting the surrounding normal tissue from radiation injury (or both). The laboratory is investigating the use of [60]fullerene derivatives that scavenge free radicals as potential radioprotectors and/or radiomitigators.
While these projects and the associated research methodologies and instrumentation provide a glimpse of scientific forays in the laboratory, the greatest resource offered by the laboratory is the intellectual capital of an array of scientists from varied, often non-intersecting, disciplines including organic chemistry, materials science, nanotechnology, cancer biology, radiation biology, immunology, radiation physics, and translational medicine. This is fortified by the current composition of the laboratory that includes two assistant professors, three postdoctoral fellows, three clinical graduates, and three students all working coordinately to advance novel nanotechnology applications in cancer and novel one-two punch strategies to eliminate recalcitrant cancers.
Team Members
- Sunil Krishnan, MD Professor and Principal Investigator
- Yuri Mackeyev, PhD Assistant Professor
- Geraldine Raja, PhD Assistant Professor
- Okan Tezcan, PhD Postdoctoral fellow
- Iona Hill, PhD Postdoctoral fellow
- Khadijeh Koushki, PhD Postdoctoral fellow
- Belal Abousaida, MD, PhD Research assistant
- Mariam Elsharnoby, MD Research assistant
- Muhammad Shohayeb, MD Research assistant
- Onur Sahin, PhD Medical student
- Holden Wagner Medical student
- Emily Han Undergraduate student
Recent Publications
- Rauta PR, Mackeyev Y, Sanders K, Kim JBK, Gonzalez VV, Zahra Y, Shohayeb MA, Abousaida B, Vijay GV, Tezcan O, Derry P, Liopo AV, Zubarev ER, Carter R, Singh P, Krishnan S. Pancreatic tumor microenvironmental acidosis and hypoxia transform gold nanorods into cell-penetrant particles for potent radiosensitization. Sci Advances 2022 Nov; 8: eabm9729
- Raghuram S, Mackeyev Y, Symons J, Zahra Y, Gonzalez V, Mahadevan KK, Requejof KI, Liopo A, Derry P, Zubarev E, Sahin O, Kim JB-K, Singh PK, Cho S, Krishnan S. Uncloaking cell-impermeant gold nanorods via tumor microenvironmental cathepsin B facilitates cancer cell penetration and potent radiosensitization. Biomaterials 2022 Dec; 291:121887.
- Roy I, Krishnan S, Kabashin AV, Zavestovskaya IN, Prasad PN. Transforming Nuclear Medicine with Nanoradiopharmaceuticals. ACS Nano. 2022 Mar 16. doi: 10.1021/acsnano.1c10550.
- Bhattarai S, Mackeyev Y, Venkatesulu BP, Krishnan S, Singh PK. CXC chemokine receptor 4 (CXCR4) targeted gold nanoparticles potently enhance radiotherapy outcomes in breast cancer. Nanoscale. 2021 Nov 25;13(45):19056-19065.
- Kim JB, Mackeyev Y, Raghuram S, Cho SH, Krishnan S. Synthesis and characterization of gadolinium-decorated [60]fullerene for tumor imaging and radiation sensitization. Int J Radiat Biol. 2021 Jan 21:1-13.
- Sahin O, Meiyazhagan A, Ajayan PM, Krishnan S. Immunogenicity of Externally Activated Nanoparticles for Cancer Therapy. Cancers (Basel). 2020 Nov 28;12(12):E3559.
- Schuemann J, Bagley AF, Berbeco R, Bromma K, Butterworth KT, Byrne HL, Chithrani BD, Cho SH, Cook JR, Favaudon V, Gholami YH, Gargioni E, Hainfeld JF, Hespeels F, Heuskin AC, Ibeh UM, Kuncic Z, Kunjachan S, Lacombe S, Lucas S, Lux F, McMahon S, Nevozhay D, Ngwa W, Payne JD, Penninckx S, Porcel E, Prise KM, Rabus H, Ridwan SM, Rudek B, Sanche L, Singh B, Smilowitz HM, Sokolov KV, Sridhar S, Stanishevskiy Y, Sung W, Tillement O, Virani N, Yantasee W, Krishnan S. Roadmap for metal nanoparticles in radiation therapy: current status, translational challenges, and future directions. Phys Med Biol. 2020 Oct 22;65(21):21RM02.
- Ayala Orozco C, Liu D, Li Y, Alemany LB, Pal R, Krishnan S, Tour JM. Visible-Light-Activated Molecular Nanomachines Kill Pancreatic Cancer Cells. ACS Appl Mater Interfaces. 2020 Jan 8;12(1):410-417.
Contact the Lab
Email: [email protected]
Office phone: (713) 500-2479