Pediatric Translational Neuroscience Laboratory
Overview
The Pediatric Translational Neuroscience Laboratory led by Peter H. Yang, MD is a clinically oriented research enterprise dedicated to improving the lives of young patients with complex neurosurgical diseases, such as hydrocephalus and craniosynostosis, that have ongoing challenges in management and varied outcomes. Our mission is to improve the understanding and treatment of diseases that primarily affect neonates and infants, where even small improvements in outcome can have significant lifelong benefits. We strive to achieve a bedside-to-bench-to-bedside approach where we identify important real-world clinical problems, implement cutting-edge biotechnology to discover solutions, and translate novel therapies directly centered on improving clinical outcomes.
Dr. Yang is a pediatric neurosurgeon who studied biomedical engineering at Johns Hopkins University and medicine at Columbia University College of Physicians and Surgeons. He completed his neurosurgery residency and pediatric neurosurgery fellowship at Washington University in St. Louis School of Medicine/St. Louis Children’s Hospital. His clinical expertise includes hydrocephalus, congenital brain and spine disorders, and vascular malformations, complementing the ongoing research efforts in the laboratory.
Inquiries from students (undergraduate, medical, and graduate) and postdoctoral researchers are highly encouraged.
CURRENT PROJECTS
Human Organoid Modeling of Pediatric Posthemorrhagic Hydrocephalus
Premature infants are at high risk of experiencing germinal matrix hemorrhage/intraventricular hemorrhage which leads to significant complications including neurodevelopmental delay and posthemorrhagic hydrocephalus (PHH) in those who survive. The pathophysiology of PHH is complex and still being understood, but it is thought to be due to an imbalance between cerebrospinal fluid (CSF) production and absorption, leading to increased intracranial pressure and further neurological deterioration. Modern day treatment options are limited and consist of surgical techniques aimed at diverting CSF (such as shunting or endoscopic surgery), rather than addressing the underlying biological mechanisms of disease. Recent work has pointed towards the choroid plexus, a specialized CSF-secreting epithelium within the ventricles, as a structure that may play a central role in PHH pathogenesis, making it an attractive target to study and to test non-surgical (i.e., pharmacogenetic) treatments. However, studying diseased human choroid plexus is exceedingly difficult and risky, and using animal models alone may not fully translate into future human treatments. This gap in knowledge necessitates an intermediary testing platform. Therefore, we are developing a 3D cell culture model of human PHH using choroid plexus organoids derived from human induced pluripotent stem cells to study human-specific pathophysiology and human-specific targets to treat PHH. These organoids form 3D cysts filled with CSF visible to the naked eye. We are using ultra-precise injection and extraction techniques to induce injury and study biological responses within the CSF and choroid plexus cells. These versatile in vitro platforms will be used to conduct high throughput pre-clinical pharmacogenomic screening experiments to treat or even prevent PHH.
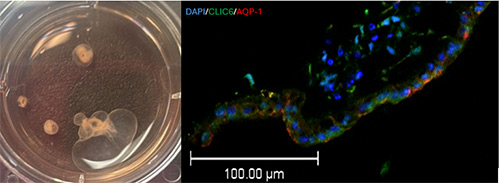
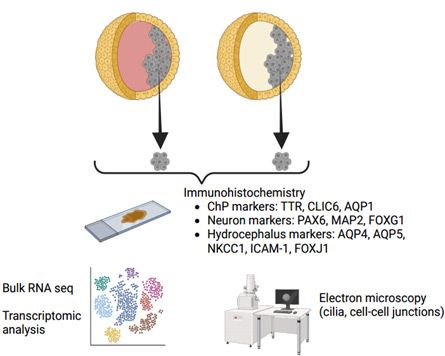
Human CSF Banking and Advanced Proteomics in Pediatric Hydrocephalus
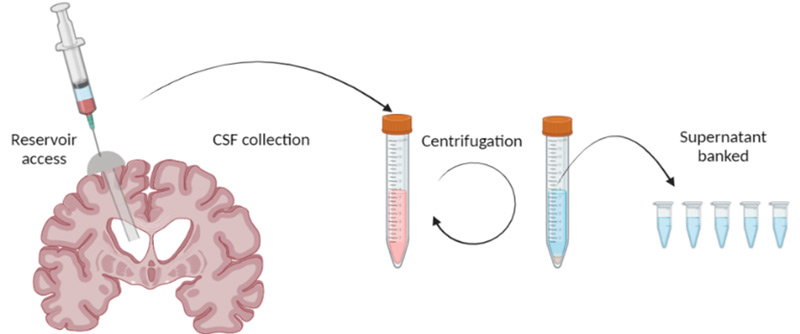
The composition of CSF varies greatly during brain development and in disease, but its exact role in human health is likely multifactorial and still being actively explored in translational research. Modern advancements in proteomics can now elucidate the highly complex protein composition within CSF, which could help identify novel biomarkers and therapeutic targets in neurological diseases. Our pediatric patients with hydrocephalus often undergo multiple neurosurgical procedures to remove CSF, which is normally discarded. We have established a robust human CSF collection pipeline to bank discarded CSF samples from patients undergoing neurosurgical procedures. In combination with detailed clinical information, these CSF samples are being analyzed using highly sophisticated protein detection techniques and advanced bioinformatics to correlate CSF biomarkers with meaningful clinical endpoints to identify new opportunities for diagnosis and treatment.
Noninvasive Intracranial Pressure Monitoring in Neonates (clinical trial)
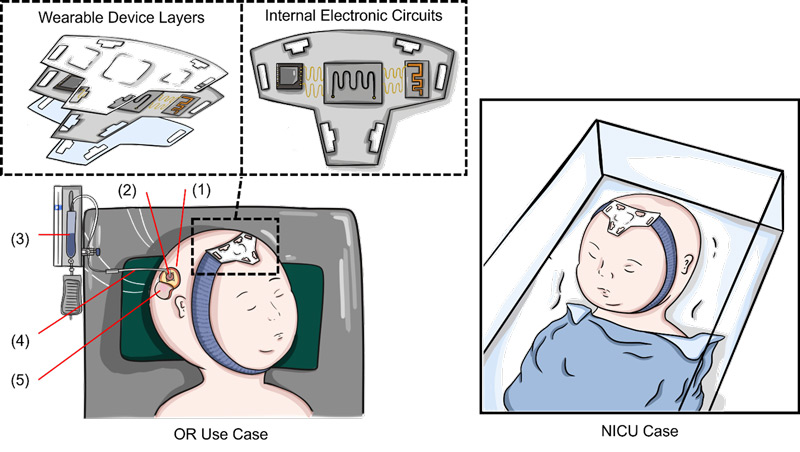
The anterior fontanelle in infants, or the “soft spot,” is a region on the head between the bilateral frontal and parietal bones that has not yet ossified. Palpation of the anterior fontanelle is a commonly utilized maneuver on physical examination to estimate the intracranial pressure in infants. However, this technique is not quantitative, requires clinical experience, and is subject to interrater variability. We are running a clinical trial with our bioengineering colleagues at Rice University to test an easy-to-use soft electronic headband containing a sensitive pressure sensor to continuously monitor intracranial pressure in infants at risk for elevated intracranial pressure/hydrocephalus.
Predictive 4D Modeling of the Growing Skull in Pediatric Craniosynostosis
Craniosynostosis is the premature fusion of any of the skull bones at the junctions, or suture lines, which can result in abnormal head shape and restricted brain growth. In infants who are a few months old and have yet to experience significant head growth, the minimally invasive options available today are to 1) surgically remove a strip of bone containing the abnormal suture and then to 2) use either external bracing (daily helmet therapy) or internal expansion (temporarily implanted springs) to reshape the head as the child grows. In current practice, caregiver preference and institutional experience tend to guide management rather than a precise understanding of the unique biomechanical forces at play for each individual patient as he or she grows. In collaboration with our Pediatric Craniofacial Surgery team at UT and bioengineering colleagues at Rice, we are performing 4D modeling of biomechanical forces in growing infant skulls to predict responses to minimally invasive surgery in a patient-specific manner.
TEAM
- Peter H. Yang, MD – Assistant Professor of Pediatric Neurosurgery
- Dr. Yang’s research is motivated by real-world clinical challenges that young patients face and the desperate need for better understanding and treatment of neurological disorders. His interests include developing tissue engineering platforms to investigate central nervous system development and bringing novel biotechnologies and therapeutics from bench to bedside to improve patient outcomes.
- Debasish Halder, PhD – Senior Program Manager, Research
- Dr. Halder completed his PhD in biochemistry from Hanyang University, and has years of research and scientific experience in neurological disorders, inflammatory diseases, and cancer. With a multidisciplinary background, his work focuses on stem-cell and 3D organoid-based translational research, aiming to bridge fundamental science with clinical applications to drive innovative therapeutic strategies. He is highly skilled in a range of advanced research methodologies, including high-content imaging, molecular and cell biology techniques, biochemical and cell-based assays, CRISPR/Cas9 gene editing, and in vivo disease modeling. His research is dedicated to uncovering the complex mechanisms underlying neurological disorders through cutting-edge experimental approaches.
- Bangning Yu, MD, PhD – Clinical Trial Program Manager
- Anik Banerjee, PhD – Postdoctoral Research Fellow
- William E. Johnson, BS – Research Associate
COLLABORATORS
- Manish N. Shah, MD – Associate Professor of Pediatric Neurosurgery
- Louise D. McCullough, MD, PhD – Professor of Neurology
- Andrea Becerra-Calixto, PhD – Research Scientist
- Raudel Avila, PhD – Assistant Professor of Mechanical Engineering
- Matthew Greives, MD – Associate Professor of Pediatric Plastic Surgery
- Danielle Sobol, MD – Assistant Professor of Pediatric Plastic Surgery
SELECTED PUBLICATIONS
Yang, Peter H., Nathan Wulfekammer, Amanda V. Jenson, Elliot G. Neal, Stuart Tomko, John Zempel, Peter Brunner, Sean D. McEvoy, Matthew D. Smyth, and Jarod L. Roland. “Safety and accuracy of stereoelectroencephalography for pediatric and young adult patients with prior craniotomy.” Journal of Neurosurgery: Pediatrics 34, no. 5 (2024): 526-536.
Yang, Peter H., John S. Schneider, Michael R. Chicoine, Albert H. Kim, and David D. Limbrick. “Endoscopic endonasal optic nerve decompression in children younger than 2 years old with congenital optic canal stenosis: illustrative cases.” Journal of Neurosurgery: Case Lessons 8, no. 2 (2024).
Yang, Peter H., Sasidhar Karuparti, Kaamya Varagur, Dimitrios Alexopoulos, Ron W. Reeder, Rachel E. Lean, Cynthia E. Rogers, David D. Limbrick, Christopher D. Smyser, and Jennifer M. Strahle. “Association of germinal matrix hemorrhage volume with neurodevelopment and hydrocephalus.” Journal of Neurosurgery: Pediatrics 1, no. aop (2024): 1-10.
Ramagiri, Sruthi, Shelei Pan, Dakota DeFreitas, Peter H. Yang, Dhvanii K. Raval, David F. Wozniak, Prabagaran Esakky, and Jennifer M. Strahle. “Deferoxamine prevents neonatal posthemorrhagic hydrocephalus through choroid plexus-mediated iron clearance.” Translational Stroke Research 14, no. 5 (2023): 704-722.
Pan, Shelei, Peter H. Yang, Dakota DeFreitas, Sruthi Ramagiri, Peter O. Bayguinov, Carl D. Hacker, Abraham Z. Snyder et al. “Gold nanoparticle-enhanced X-ray microtomography of the rodent reveals region-specific cerebrospinal fluid circulation in the brain.” Nature communications 14, no. 1 (2023): 453.
Miller, Brandon A., Shelei Pan, Peter H. Yang, Catherine Wang, Amanda L. Trout, Dakota DeFreitas, Sruthi Ramagiri, Scott D. Olson, and Jennifer M. Strahle. “Modeling neonatal intraventricular hemorrhage through intraventricular injection of hemoglobin.” Journal of visualized experiments: JoVE 186 (2022): 10-3791.
Yang, Peter H., Alison Almgren-Bell, Hongjie Gu, Anna V. Dowling, Sangami Pugazenthi, Kimberly Mackey, Esther B. Dupépé, and Jennifer M. Strahle. “Etiology-and region-specific characteristics of transependymal cerebrospinal fluid flow.” Journal of Neurosurgery: Pediatrics 30, no. 4 (2022): 437-447.
Lee, Angela, Samuel Cortez, Peter Yang, Diane Aum, Prapti Singh, Catherine Gooch, and Matthew Smyth. “Neonatal hydrocephalus: an atypical presentation of malignant infantile osteopetrosis.” Child’s Nervous System (2021): 1-9.
Kennedy, Benjamin C., Michael M. McDowell, Peter H. Yang, Caroline M. Wilson, Sida Li, Todd C. Hankinson, Neil A. Feldstein, and Richard CE Anderson. “Pial synangiosis for moyamoya syndrome in children with sickle cell anemia: a comprehensive review of reported cases.” Neurosurgical focus 36, no. 1 (2014): E12.
See full list of Google Scholar citations
CONTACT US
Peter H. Yang, MD, Assistant Professor of Pediatric Neurosurgery
6431 Fannin St., MSB 5.138
Houston, TX 77030