Antibody Specificity
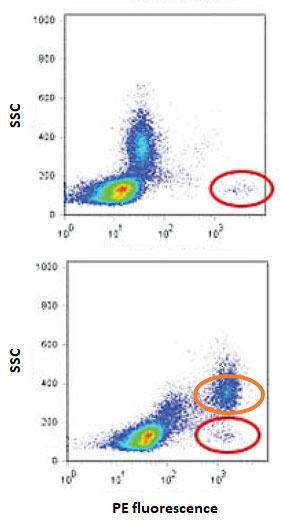
Fig. 1. Same antibody clone (PE conjugated mouse anti-human CD34, clone 581) from two different vendors showing distinctly different staining patterns. Cell population of interest circled with red, cell population showing unwanted binding to one of the products circled with orange. Figure modified from Hulspas (2009).
Accurate flow cytometry analysis depends on the specificity of the antibodies used. Unfortunately, majority of commercially available antibodies are poorly validated and it is up to the end user to make sure that the antibodies work as expected. Nearly 50% of the antibodies submitted to the Human Leucocyte Differentiation Antigen Workshops fail to function as intended!
Typical problems in flow cytometry include unspecific binding and specific cross-reactivity with off targets (resulting in false positives; Fig. 1.) as well as lack of reactivity with the stated target antigen (resulting in false negatives). Note also that many antibodies used in e.g. Western blotting are raised against linear epitopes that may not be present/exposed in living cells whereas common flow cytometry sample processing steps such as enzymatic digestion and fixation/permeabilization can alter the native epitopes (or the antibodies and fluorophores themselves).
Controls to Validate Antibody Specificity:
There are several different controls that can be used to validate the specificity of your antibodies.
- Positive control cells
- Primary cells known to express the antigen of interest (sometimes only after stimulation)
- Cell lines transfected to express the antigen of interest (fusion tags such as GFP or HA can give an independent way to confirm expression when no previously validated antibody is available for direct comparison)
- Negative control cells
- Primary cells or mock transfected cell line known to not express the antigen of interest
- Targeted knock out cell lines (or cells from transgenic animals) where the antigen expression has been specifically eliminated
- Competition controls
- Excess unlabeled antibody used to block binding of labeled version of the same antibody
- Excess soluble antigen used to block antibody binding to target cells
- Generic blocking agents
- Fc block and cyanine dye block (especially for monocytes and macrophages)
- Polymer dye specific block (when using two or more BD Horizon Brilliant™ or Super Bright dyes together)
- Secondary only control
- Staining without primary antibody
Additional Information:
- Hulspas (2009) Considerations for the control of background fluorescence in clinical flow cytometry. Cytometry B 76B:355–364, doi: 10.1002/cyto.b.20485
- Bradbury and Plückthun (2015) Reproducibility: Standardize antibodies used in research. Nature 518, 27–29, doi:10.1038/518027a
- Reproducibility Crisis and Antibody Validation of Flow Cytometry, CytoU webinar