Antibody Titration
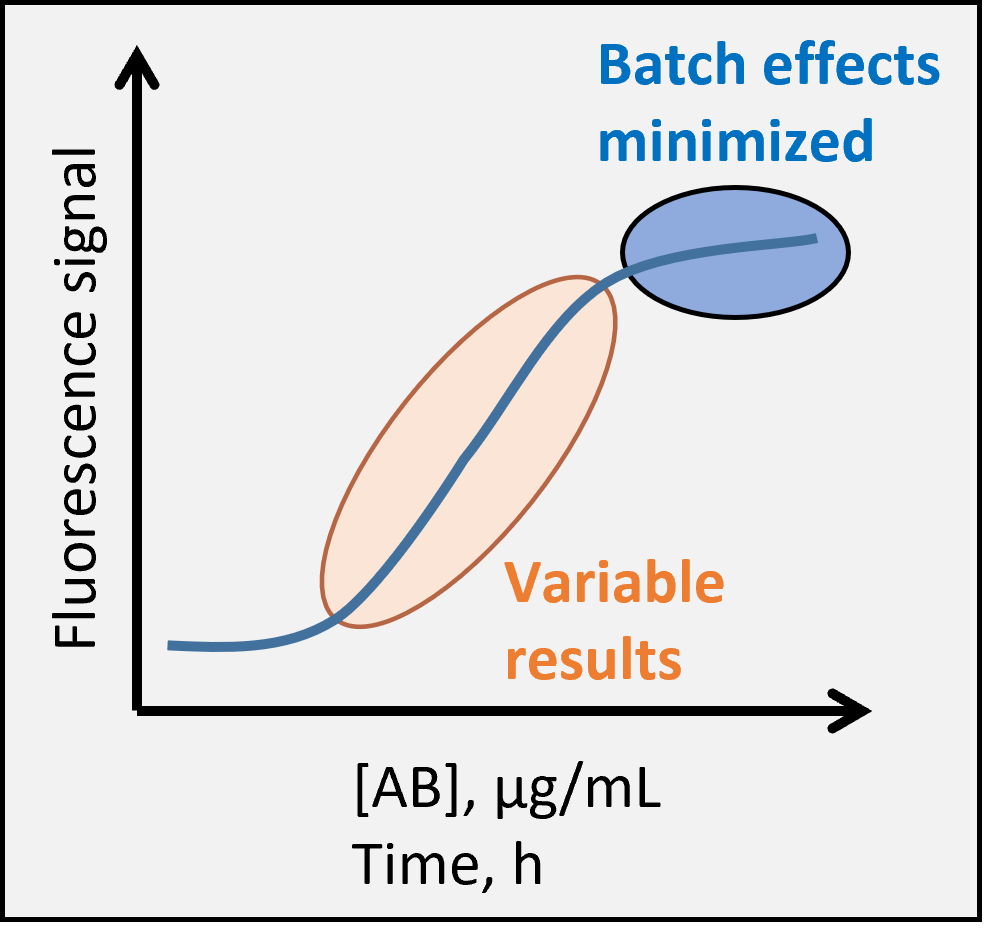
Fig. 1. Robust assay requires consistent staining.
The correct antibody concentration is very important to ensure best resolution and consistent results in flow cytometry analysis. Too little antibody and the specific positive signal remains weak and susceptible to small changes in staining conditions, too much antibody and the unspecific background signal starts to increase (Fig. 1). Furthermore, the manufacturer recommendations often lead to great excess of antibody in the staining protocol and many times it is possible to save money and reagents by doing your own titration. Note that typical staining protocols use high antibody concentrations in order to achieve near complete epitope saturation in 30-60 minutes. But especially if your cell isolation takes a long time, it may be advantageous to stain the samples overnight instead – with less antibody – and analyze the samples next day.
Titration Tips:
- Titrate antibodies in the exact same conditions as your experiment (temperature, incubation time, volume, cell type and number)
- Repeat titration for each new antibody lot and after any significant change in the sample preparation/staining process
- Titrate each antibody separately, but a second antibody (with fixed concentration) can be added as needed to help resolve the population of interest
- Minimize signal noise by including viability dye and Fc block, and by gating on singlets
- Start with 5 µg/mL (or manufacturer recommended dilution if concentration is unknown; you can always call the company and ask) and make at least 6-point 1:2 dilution series plus unstained control
Finding the Optimal Concentration:
-
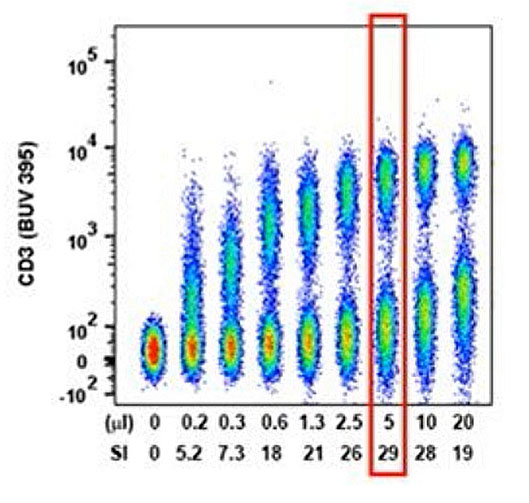
Fig. 2. Concatenated raw data with calculated separation index from a typical antibody titration experiment. 5 µL of antibody per reaction has already saturated practically all the CD3 epitopes and any increase in staining concentration would only hinder detection of dim populations. Figure from Cytometry A. 2016 May;89(5):427-9. doi: 10.1002/cyto.a.22808
Collect data to include a minimum of 1,000 positive events for each concentration
- Adjust gates separately for each concentration in the dilution series to correctly identify negatives and positives
- Measure Median Fluorescence Intensity for negatives and positives and determine the spread in the negative population
- Calculate Separation Index (or alternatively Staining Index which uses standard deviation instead of percentiles to determine spread) for each condition and identify the optimal antibody concentration
- The concentration giving highest Separation Index will result in robust assays; small pipetting errors or 5x variations in total cell numbers will have minimal effect on staining
- Final assay concentration may be decreased below saturation level if you need to minimize fluorescence spillover in complex multicolor panels and the negatives and positives still remain well separated
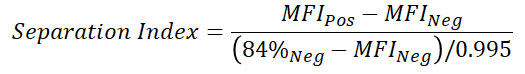
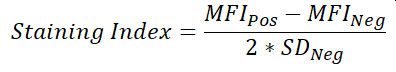
Additional Information:
- Telford (2009) Green fiber lasers: An alternative to traditional DPSS green lasers for flow cytometry. Cytometry A. 75A:1031-1039, doi:10.1002/cyto.a.20790
- Hulspas (2010) Titration of fluorochrome-conjugated antibodies for labeling cell surface markers on live cells. Curr Protoc Cytom. 54:6.29.1-6.29.9, doi: 10.1002/0471142956.cy0629s54