Fc Blocking
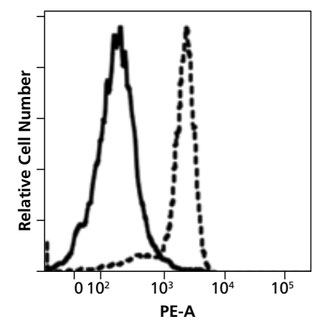
Fig. 1. Isotype control binding to the monocyte fraction of human PBMCs with (solid line) and without (dashed line) Fc blocking. Image from Human BD Fc Block™ technical data sheet.
Flow cytometry utilizes fluorescently labeled antibodies to bind and identify specific cellular subsets. The specificity of the binding relies on the unique variable regions of each antibody clone. In contrast, it is the common Fc part of the antibody molecules that interacts with a plethora of immune cells (such as B lymphocytes, dendritic cells, monocytes, macrophages, NKs, neutrophils, eosinophils, human platelets, mast cells and basophils) responsible for the in vivo observed effector functions. These cells express a variety of Fc receptors on their surface and can therefore generate increased background or even false positive subpopulations in flow cytometry analysis. Monocytes and macrophages are especially notorious in this regard (Fig. 1).
The unwanted antibody binding to Fc receptors can be avoided by using recombinant detection antibodies (e.g. Fab fragments, REAfinity™ antibodies), or more commonly, be blocked by saturating the receptors prior to staining the cells with labeled antibodies. To keep the receptors saturated the blocker is left in place during the antibody incubation. Potential blocking reagents include 1) specific anti-Fc receptor antibodies, 2) excess purified IgG, and 3) excess (unpurified) IgG in the form of adult serum.
Note that fetal bovine serum commonly included in the staining buffer recipes has too low IgG content and will not block the Fc receptors.
Isotype controls (labeled antibodies with irrelevant specificity) can be used to evaluate the effectiveness of the Fc blocking protocol. The use of isotype controls for gating purposes, however, is not recommended.
Additional Information:
- Rosales (2017) Fcγ receptor heterogeneity in leukocyte functional responses. Front. Immunol, doi:10.3389/fimmu.2017.00280
- Andersen et al. (2016) Elimination of erroneous results in flow cytometry caused by antibody binding to Fc receptors on human monocytes and macrophages. Cytometry A. 89:1001-1009, doi: 10.1002/cyto.a.22995