Clinical Trials
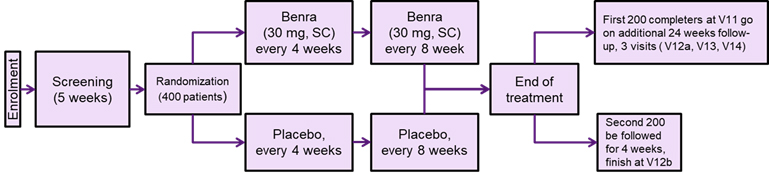
The Effect of Benralizumab on Nasal Polyp Burden in Patients With Chronic Sinusitis and Bilateral Nasal Polyposis with and without Concomitant Asthma
The primary objective of this study is to evaluate the efficacy of benralizumab (anti-IL5) on polyp size.
Main inclusion criteria:
- A minimum bilateral nasal polyp score of 4 out of a maximum score of 8 (with a unilateral score of at least 2 for each nostril) despite completion of a prior INCS treatment for at least 8 weeks before screening.
- Presence of at least two of the following symptoms prior to screening: Nasal obstruction/blockage/congestion or discharge (anterior/posterior nasal drip), facial pain or pressure, and reduction or loss of smell
- age 18 through 75 years with a minimum weight of 40 kg
Main exclusion criteria:
- Sinus surgery within 3 months
- Oral prednisone use within 4 weeks
- Asthma exacerbation within 4 weeks
A Prospective, Multi-Center Registry of Subjects Treated with the ClariFix™ Cryotherapy Device
The primary objective of this study capture observational data on individuals treated with the ClariFix device. The ClariFix device is a handheld cryosurgical device that provides focal, controlled freezing to the target tissue. It is used in the nasal passageway of patients with chronic rhinitis to address symptoms of rhinorrhea and nasal congestion.
Main inclusion criteria:
- Subject is scheduled to receive treatment with the ClariFix device in accordance with the ClariFix Instructions for Use (LBL-0384).
- Subject is able to provide consent and willing to adhere to the study visit schedule.
Main exclusion criteria:
- Subject is scheduled to have additional treatment and/or procedures completed at the same time of treatment with the ClariFix device.
- Subject is scheduled to have additional nasal or sinus treatments and/or procedures completed within three (3) months of treatment with the ClariFix device.